Abstract
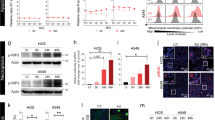
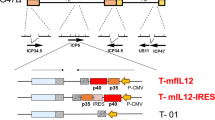
To enhance further the safety and efficacy of oncolytic vaccinia virus, we have developed a new virus with targeted deletions of three viral genes encoding thymidine kinase and antiapoptotic/host range proteins SPI-1 and SPI-2 (vSPT). Infection of human and murine tumor cell lines yielded nearly equivalent or a log lower virus recovery in comparison to parental viruses. Viral infection activated multiple caspases in cancer cells but not in normal cells, suggesting infected cells may die via different pathways. In tumor-bearing mice, vSPT recovery from MC38 tumor was slightly reduced in comparison to two parental viruses. However, no virus was recovered from the brains and livers of mice injected with vSPT in contrast to control viruses. vSPT demonstrated significantly lower pathogenicity in nude mice. Systemic delivery of vSPT showed significant tumor inhibition in subcutaneous MC38 tumor, human ovarian A2780 and murine ovarian MOSEC carcinomatosis models; however, the tumor inhibition by vSPT was reduced compared with parental viruses. These results demonstrated that although deletion of these three viral genes further enhanced tumor selectivity, it also weakened the oncolytic potency. This study illustrates the complexity of creating a tumor-selective oncolytic virus by deleting multiple viral genes involved in multiple cellular pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nakamura T, Russell SJ . Oncolytic measles viruses for cancer therapy. Exp Opin Biol Ther 2004; 4: 1685–1692.
Aghi M, Martuza RL . Oncolytic viral therapies – the clinical experience. Oncogene 2005; 24: 7802–7816.
Davis JJ, Fang B . Oncolytic virotherapy for cancer treatment: challenges and solutions. J Gene Med 2005; 7: 1380–1389.
Everts B, van der Poel HG . Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther 2005; 12: 141–161.
O'Shea CC . Viruses – seeking and destroying the tumor program. Oncogene 2005; 24: 7640–7655.
Parato KA, Senger D, Forsyth PA, Bell JC . Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005; 5: 965–976.
Thorne SH, Bartlett DL, Kirn DH . The use of oncolytic vaccinia viruses in the treatment of cancer: a new role for an old ally? Curr Gene Ther 2005; 5: 429–443.
Zeh HJ, Bartlett DL . Development of a replication-selective, oncolytic poxvirus for the treatment of human cancers. Cancer Gene Ther 2002; 9: 1001–1012.
Guo ZS, Bartlett DL . Vaccinia as a vector for gene delivery. Expert Opin Biol Ther 2004; 4: 901–917.
Shen Y, Nemunaitis J . Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther 2005; 11: 180–195.
Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA . Smallpox vaccination: a review, Part II. Adverse events. Clin Infect Dis 2003; 37: 251–271.
Peplinski GR, Tsung K, Casey MJ, Meko JB, Fredrickson TN, Buller RM et al. In vivo murine tumor gene delivery and expression by systemic recombinant vaccinia virus encoding interleukin-1beta. Cancer J Sci Am 1996; 2: 21–27.
Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR et al. Vaccinia as a vector for tumor directed gene therapy: biodistribution of a thymidine kinase deleted mutant. Cancer Gene Ther 2000; 7: 66–73.
Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 2004; 22: 313–320.
McCart JA, Mehta N, Scollard D, Reilly RM, Carrasquillo JA, Tang N et al. Oncolytic vaccinia virus expressing the human somatostatin receptor SSTR2: molecular imaging after systemic delivery using 111In-pentetreotide. Mol Ther 2004; 10: 553–561.
McFadden G . Poxvirus tropism. Nature Rev Microbiol 2005; 3: 201–213.
Chang E, Chalikonda S, Friedl J, Xu H, Phan GQ, Marincola FM et al. Targeting vaccinia to solid tumors with local hyperthermia. Hum Gene Ther 2005; 16: 435–444.
McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 2001; 61: 8751–8757.
Gnant MFX, Puhlmann M, Alexander HR, Bartlett DL . Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-fluorocytosine leads to tumor specific gene expression and prolongation of survival in mice. Cancer Res 1999; 59: 3396–3404.
Naik AM, Chalikonda S, McCart JA, Xu H, Guo ZS, Langham G et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther 2006; 17: 31–45.
Liu TC, Kirn D . Viruses with deletions in antiapoptotic genes as potential oncolytic agents. Oncogene 2005; 24: 6069–6079.
Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PGW et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. J Biol Chem 2001; 276: 33293–33296.
Ali AN, Turner PC, Brooks MA, Moyer RW . The Spi-1 gene of rabbitpox virus determines host-range and is required for hemorrhagic pock formation. Virology 1994; 202: 305–314.
Brooks MA, Ali AN, Turner PC, Moyer RW . A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J Virol 1995; 69: 7688–7698.
Kettle S, Blake NW, Law KM, Smith GL . Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40 K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology 1995; 206: 136–147.
Macen JL, Garner RS, Musy PY, Brooks MA, Turner PC, Moyer RW et al. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc Natl Acad Sci USA 1996; 93: 9108–9113.
Guo ZS, Naik A, O'Malley ME, Popovic P, DeMarco R, Hu Y et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and anti-apoptosis genes SPI-1 and SPI-2. Cancer Res 2005; 65: 9991–9998.
Igney FH, Krammer PH . Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277–288.
Taneja S, MacGregor J, Markus S, Ha S, Mohr I . Enhanced antitumor efficacy of a herpes simplex virus mutant isolated by genetic selection in cancer cells. Proc Natl Acad Sci USA 2001; 98: 8804–8808.
Ito H, Aoki H, Kuhnel P, Kondo Y, Kubicka S, Wirth T et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst 2006; 98: 625–636.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304: 1500–1502.
Essajee S, Kaufman HL . Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther 2004; 4: 575–588.
Woo Y, Adusumilli PS, Fong Y . Advances in oncolytic viral therapy. Curr Opin Investig Drugs 2006; 7: 549–559.
Puhlmann M, Gnant M, Brown CK, Alexander HR, Bartlett DL . Thymidine kinase deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor directed gene therapy. Hum Gene Ther 1999; 10: 649–657.
McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR et al. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther 2000; 7: 1217–1223.
Kirn D, Niculescu-Duvaz I, Hallden G, Springer CJ . The emerging fields of suicide gene therapy and virotherapy. Trends Mol Med 2002; 8: S68–S73.
Mastrangelo MJ, Lattime EC . Virotherapy clinical trials for regional disease: in situ immune modulation using recombinant poxvirus vectors. Cancer Gene Ther 2002; 9: 1013–1021.
Legrand FA, Verardi PH, Chan KS, Peng Y, Jones LA, Yilma TD . Vaccinia viruses with a serpin gene deletion and expressing IFN-gamma induce potent immune responses without detectable replication in vivo. Proc Natl Acad Sci USA 2005; 102: 2940–2945.
Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 2006; 14: 361–370.
Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res 2006; 66: 1105–1113.
Thorne SH, Negrin RS, Contag CH . Synergistic antitumor effects of immune cell-viral biotherapy. Science 2006; 311: 1780–1784.
Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000; 21: 585–591.
Acknowledgements
We thank Katherine F Roby at University of Kansas for providing the MOSEC cell line, Teresa Whiteside at the University of Pittsburgh Cancer Institute for providing normal human primary fibroblasts. We also thank Charlie Brown, Michael Gorry and Eric Dong for suggestions and critical reading of the manuscript. This study was supported in part by National Institutes of Health Grant CA100415.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Guo, Z., O'Malley, M. et al. A new recombinant vaccinia with targeted deletion of three viral genes: its safety and efficacy as an oncolytic virus. Gene Ther 14, 638–647 (2007). https://doi.org/10.1038/sj.gt.3302914
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302914