Abstract
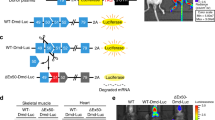
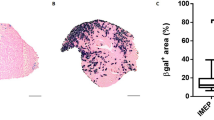
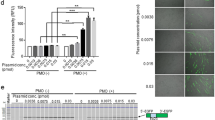
Muscular dystrophies are a genetically and phenotypically heterogeneous group of degenerative muscle diseases. A subset of them are due to genetic deficiencies in proteins which form the dystrophin-associated complex at the membrane of the myofibers. In this report, we utilized recombinant adeno-associated virus containing a U7 cassette carrying an antisense sequence aimed at inducing exon skipping of the dystrophin gene or containing the α-sarcoglycan gene to alleviate the dystrophic phenotype of the mdx and Sgca-null mice, respectively. As these diseases are characterized by cycle of degeneration/regeneration, we postulated that a reporter gene coadministered at the time of the treatment would make it possible to follow the extent of muscle repair. We observed that the murine secreted alkaline phosphatase (muSeAP) level was very much lower in these animal models than in normal mice. Upon treatment of the dystrophic muscle by gene transfer, the level of muSeAP was restored and correlated with the expression of the therapeutic transgene and with the level of muscle improvement. The system described here provides a simple and noninvasive procedure for monitoring the outcome of a therapeutic strategy involving cell survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Straub V, Campbell KP . Muscular dystrophies and the dystrophin–glycoprotein complex. Curr Opin Neurol 1997; 10: 168–175.
Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM . Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 1986; 323: 646–650.
Hoffman EP, Brown Jr RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Noguchi S, McNally EM, Ben Othmane K, Hagiwara Y, Mizuno Y, Yoshida M et al. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science 1995; 270: 819–822.
Lim LE, Duclos F, Broux O, Bourg N, Sunada Y, Allamand V et al. Beta-sarcoglycan: characterization and role in limb-girdle muscular dystrophy linked to 4q12. Nat Genet 1995; 11: 257–265.
Bonnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E et al. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet 1995; 11: 266–273.
Nigro V, de Sa Moreira E, Piluso G, Vainzof M, Belsito A, Politano L et al. Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat Genet 1996; 14: 195–198.
Roberds SL, Leturcq F, Allamand V, Piccolo F, Jeanpierre M, Anderson RD et al. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell 1994; 78: 625–633.
Karpati G, Hilton-Jones D, Griggs RC . Disorders of Voluntary Muscle. Cambridge, New York: Cambridge University Press, 2001.
Badalamente MA, Stracher A . Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve 2000; 23: 106–111.
Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE . Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 1996; 384: 349–353.
Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002; 420: 418–421.
Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL . Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest 1999; 104: 375–381.
Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 1993; 361: 647–650.
Rando TA, Disatnik MH, Zhou LZ . Rescue of dystrophin expression in mdx mouse muscle by RNA/DNA oligonucleotides. Proc Natl Acad Sci USA 2000; 97: 5363–5368.
Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM . Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 1989; 337: 176–179.
Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999; 401: 390–394.
Wang B, Li J, Xiao X . Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA 2000; 97: 13714–13719.
Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A et al. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 1991; 352: 815–818.
Fabb SA, Wells DJ, Serpente P, Dickson G . Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet 2002; 11: 733–741.
Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 2004; 306: 1796–1799.
Dressman D, Araishi K, Imamura M, Sasaoka T, Liu LA, Engvall E et al. Delivery of alpha- and beta-sarcoglycan by recombinant adeno-associated virus: efficient rescue of muscle, but differential toxicity. Hum Gene Ther 2002; 13: 1631–1646.
Cordier L, Hack AA, Scott MO, Barton-Davis ER, Gao G, Wilson JM et al. Rescue of skeletal muscles of gamma-sarcoglycan-deficient mice with adeno-associated virus-mediated gene transfer. Mol Ther 2000; 1: 119–129.
Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther 2004; 10: 821–828.
Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med 1999; 5: 439–443.
Gillis JM, Deconinck N . The physiological evaluation of gene therapies of dystrophin-deficient muscles. Adv Exp Med Biol 1998; 453: 411–416; (discussion 417).
Wang M, Orsini C, Casanova D, Millan JL, Mahfoudi A, Thuillier V . MUSEAP, a novel reporter gene for the study of long-term gene expression in immunocompetent mice. Gene 2001; 279: 99–108.
Hauser MA, Robinson A, Hartigan-O'Connor D, Williams-Gregory DA, Buskin JN, Apone S et al. Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol Ther 2000; 2: 16–25.
Matsuda R, Nishikawa A, Tanaka H . Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem (Tokyo) 1995; 118: 959–964.
Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE . Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther 2000; 2: 619–623.
Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM . Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999; 73: 3994–4003.
Hauck B, Xiao W . Characterization of tissue tropism determinants of adeno-associated virus type 1. J Virol 2003; 77: 2768–2774.
Lovering RM, De Deyne PG . Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 2004; 286: C230–C238.
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL . Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 1993; 90: 3710–3714.
Lapidos KA, Kakkar R, McNally EM . The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res 2004; 94: 1023–1031.
Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA . Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol 2000; 279: C1290–C1294.
Duclos F, Straub V, Moore SA, Venzke DP, Hrstka RF, Crosbie RH et al. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J Cell Biol 1998; 142: 1461–1471.
Gillis JM . Membrane abnormalities and Ca homeostasis in muscles of the mdx mouse, an animal model of the Duchenne muscular dystrophy: a review. Acta Physiol Scand 1996; 156: 397–406.
Alderton JM, Steinhardt RA . Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 2000; 275: 9452–9460.
McCarter GC, Steinhardt RA . Increased activity of calcium leak channels caused by proteolysis near sarcolemmal ruptures. J Membr Biol 2000; 176: 169–174.
Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P . Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 2002; 158: 1089–1096.
Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther 2001; 12: 205–215.
Gillis JM . Multivariate evaluation of the functional recovery obtained by the overexpression of utrophin in skeletal muscles of the mdx mouse. Neuromuscul Disord 2002; 12 (Suppl 1): S90–S94.
Straub V, Donahue KM, Allamand V, Davisson RL, Kim YR, Campbell KP . Contrast agent-enhanced magnetic resonance imaging of skeletal muscle damage in animal models of muscular dystrophy. Magn Reson Med 2000; 44: 655–659.
Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997; 16: 270–276.
Zhang HG, Wang YM, Xie JF, Liang X, Hsu HC, Zhang X et al. Recombinant adenovirus expressing adeno-associated virus cap and rep proteins supports production of high-titer recombinant adeno-associated virus. Gene Therapy 2001; 8: 704–712.
Apparailly F, Khoury M, Vervoordeldonk MJ, Adriaansen J, Gicquel E, Perez N et al. Adeno-associated virus pseudotype 5 vector improves gene transfer in arthritic joints. Hum Gene Ther 2005; 16: 426–434.
Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther 1998; 9: 695–706.
Acknowledgements
We acknowledge the excellent expertise of the In vivo department of Généthon, especially Ludovic Arandel for animal procedures and Daniel Stockholm for help in the real-time quantitative RT-PCR experiments. We are grateful to Susan Cure for critical reading of the manuscript. We would like to thank the Howard Hughes Medical Institute (Iowa City, USA) for providing us with Sgca-null mice and Vincent Thuillier (Gencell, Vitry-sur-Seine, France) for the pVT20 plasmid. AG is the recipient of a fellowship from the Ministère de l'Éducation Nationale, de la Recherche et de la Technologie. This work was funded by the Association Française contre les Myopathies, Genopole® (Evry) and the Fondation pour la Recherche Médicale.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartoli, M., Poupiot, J., Goyenvalle, A. et al. Noninvasive monitoring of therapeutic gene transfer in animal models of muscular dystrophies. Gene Ther 13, 20–28 (2006). https://doi.org/10.1038/sj.gt.3302594
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302594
Keywords
This article is cited by
-
Rapid, scalable, and low-cost purification of recombinant adeno-associated virus produced by baculovirus expression vector system
Molecular Therapy - Methods & Clinical Development (2016)
-
A comparison of AAV strategies distinguishes overlapping vectors for efficient systemic delivery of the 6.2 kb Dysferlin coding sequence
Molecular Therapy - Methods & Clinical Development (2015)
-
Simple downstream process based on detergent treatment improves yield and in vivo transduction efficacy of adeno-associated virus vectors
Molecular Therapy - Methods & Clinical Development (2015)
-
Forelimb Treatment in a Large Cohort of Dystrophic Dogs Supports Delivery of a Recombinant AAV for Exon Skipping in Duchenne Patients
Molecular Therapy (2014)
-
AAV Genome Loss From Dystrophic Mouse Muscles During AAV-U7 snRNA-mediated Exon-skipping Therapy
Molecular Therapy (2013)