Abstract
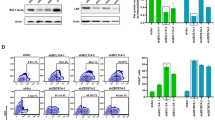
Adenoviral gene transfer to hematopoietic stem cells (HSCs)/progenitors would provide a new approach to the treatment of hematopoietic diseases and study of the hematopoietic system. We have previously reported that an adenovirus (Ad) vector composed of whole Ad serotype 35 (Ad35), which belongs to subgroup B, shows efficient gene transfer into human bone marrow CD34+ cells. However, Ad35 vector-mediated transduction into human HSCs/progenitors has not yet been fully optimized. In the present study, we have systematically examined promoter activity in the context of Ad35 vectors in human bone marrow CD34+ cells and primitive CD34+ subsets to optimize the transduction efficiency in human hematopoietic stem/progenitor cells. In the first of the transduction experiments, the improved in vitro ligation method was applied to Ad35 vector construction to allow for simple and efficient production of an E1/E3-deleted Ad35 vector. Using this method, we constructed a series of Ad35 vectors encoding the enhanced green fluorescence protein (GFP) under the control of a variety of strong viral and cellular promoters. Of the six types of promoters tested, significantly higher transduction efficiencies were achieved with the human elongation factor 1α promoter (EF1α promoter), the human cytomegalovirus (CMV) immediate-early 1 gene enhancer/β-actin promoter with β-actin intron (CA promoter), and the CMV promoter/enhancer with the largest intron of CMV (intron A) (CMVi promoter) in the human CD34+ cells and the immature subsets (CD34+CD38low/− and CD34+AC133+ subsets). In particular, the CA promoter was found to allow for the highest transduction efficiencies in both the whole human CD34+ cells and the immature hematopoietic subsets. Furthermore, the CA promoter-mediated GFP-expressing cells differentiated into progenitor cells of all lineages. These results indicate the construction of an optimized Ad35 vector backbone for efficient transduction into HSCs/progenitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Dao MA, Shah AJ, Crooks GM, Nolta JA . Engraftment and retroviral marking of CD34+ and CD34+CD38− human hematopoietic progenitors assessed in immune-deficient mice. Blood 1998; 91: 1243–1255.
Orlic D et al. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA 1996; 93: 11097–11102.
Sirven A et al. Enhanced transgene expression in cord blood CD34(+)-derived hematopoietic cells, including developing T cells and NOD/SCID mouse repopulating cells, following transduction with modified trip lentiviral vectors. Mol Ther 2001; 3: 438–448.
Kahn J et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood 2004; 103: 2942–2949.
Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, Lieber A . Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol 2000; 74: 2567–2583.
Yotnda P et al. Efficient infection of primitive hematopoietic stem cells by modified adenovirus. Gene Therapy 2001; 8: 930–937.
Rebel VI et al. Maturation and lineage-specific expression of the coxsackie and adenovirus receptor in hematopoietic cells. Stem Cells 2000; 18: 176–182.
Takahashi T et al. A potential molecular approach to ex vivo hematopoietic expansion with recombinant epidermal growth factor receptor-expressing adenovirus vector. Blood 1998; 91: 4509–4515.
Segerman A, Mei YF, Wadell G . Adenovirus types 11p and 35p show high binding efficiencies for committed hematopoietic cell lines and are infective to these cell lines. J Virol 2000; 74: 1457–1467.
Sakurai F, Mizuguchi H, Hayakawa T . Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Therapy 2003; 10: 1041–1048.
Gaggar A, Shayakhmetov DM, Lieber A . CD46 is a cellular receptor for group B adenoviruses. Nat Med 2003; 9: 1408–1412.
Segerman A et al. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol 2003; 77: 9183–9191.
Johnstone RW, Loveland BE, McKenzie IF . Identification and quantification of complement regulator CD46 on normal human tissues. Immunology 1993; 79: 341–347.
Liszewski MK, Post TW, Atkinson JP . Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol 1991; 9: 431–455.
Manchester M et al. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol 2002; 76: 6636–6642.
Fan X, Brun A, Karlsson S . Adenoviral vector design for high-level transgene expression in primitive human hematopoietic progenitors. Gene Therapy 2000; 7: 2132–2138.
Byk T, Haddada H, Vainchenker W, Louache F . Lipofectamine and related cationic lipids strongly improve adenoviral infection efficiency of primitive human hematopoietic cells. Hum Gene Ther 1998; 9: 2493–2502.
Frey BM et al. High-efficiency gene transfer into ex vivo expanded human hematopoietic progenitors and precursor cells by adenovirus vectors. Blood 1998; 91: 2781–2792.
Ramezani A, Hawley TS, Hawley RG . Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther 2000; 2: 458–469.
Mizuguchi H, Kay MA . Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther 1998; 9: 2577–2583.
Mizuguchi H, Kay MA . A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum Gene Ther 1999; 10: 2013–2017.
Krougliak V, Graham FL . Development of cell lines capable of complementing E1, E4, and protein IX defective adenovirus type 5 mutants. Hum Gene Ther 1995; 6: 1575–1586.
Sakurai F, Mizuguchi H, Yamaguchi T, Hayakawa T . Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol Ther 2003; 8: 813–821.
Foecking MK, Hofstetter H . Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene 1986; 45: 101–105.
Hao QL et al. A functional comparison of CD34 + CD38− cells in cord blood and bone marrow. Blood 1995; 86: 3745–3753.
Yin AH et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997; 90: 5002–5012.
Hao QL et al. Extended long-term culture reveals a highly quiescent and primitive human hematopoietic progenitor population. Blood 1996; 88: 3306–3313.
Guo ZS, Wang LH, Eisensmith RC, Woo SL . Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Therapy 1996; 3: 802–810.
Xu ZL et al. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene 2001; 272: 149–156.
Cheng L, Ziegelhoffer PR, Yang NS . In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA 1993; 90: 4455–4459.
Ponder KP et al. Evaluation of relative promoter strength in primary hepatocytes using optimized lipofection. Hum Gene Ther 1991; 2: 41–52.
Lizee G, Gonzales MI, Topalian SL . Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther 2004; 15: 393–404.
Kiwaki K et al. Correction of ornithine transcarbamylase deficiency in adult spf(ash) mice and in OTC-deficient human hepatocytes with recombinant adenoviruses bearing the CAG promoter. Hum Gene Ther 1996; 7: 821–830.
Okabe M et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 1997; 407: 313–319.
Araki K et al. Efficiency of recombination by Cre transient expression in embryonic stem cells: comparison of various promoters. J Biochem (Tokyo) 1997; 122: 977–982.
Kawabata K et al. Efficient gene transfer into mouse embryonic stem cells with adenovirus vectors. Mol Ther (in press).
Chung S et al. Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells 2002; 20: 139–145.
Sirena D et al. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol 2004; 78: 4454–4462.
Schroers R et al. Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp Hematol 2004; 32: 536–546.
Anderson BD, Nakamura T, Russell SJ, Peng KW . High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 2004; 64: 4919–4926.
Segerman A et al. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J Virol 2003; 77: 1157–1162.
Jin CH et al. Recombinant Sendai virus provides a highly efficient gene transfer into human cord blood-derived hematopoietic stem cells. Gene Therapy 2003; 10: 272–277.
Knaan-Shanzer S et al. Highly efficient targeted transduction of undifferentiated human hematopoietic cells by adenoviral vectors displaying fiber knobs of subgroup B. Hum Gene Ther 2001; 12: 1989–2005.
Dorrell C et al. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood 2000; 95: 102–110.
Donaldson C, Denning-Kendall P, Bradley B, Hows J . The CD34(+)CD38(neg) population is significantly increased in haemopoietic cell expansion cultures in serum-free compared to serum-replete conditions: dissociation of phenotype and function. Bone Marrow Transplant 2001; 27: 365–371.
Mizuguchi H, Kay MA, Hayakawa T . Approaches for generating recombinant adenovirus vectors. Adv Drug Deliv Rev 2001; 52: 165–176.
Seshidhar Reddy P et al. Development of adenovirus serotype 35 as a gene transfer vector. Virology 2003; 311: 384–393.
Vogels R et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77: 8263–8271.
Gao W, Robbins PD, Gambotto A . Human adenovirus type 35: nucleotide sequence and vector development. Gene Therapy 2003; 10: 1941–1949.
Shayakhmetov DM et al. A high-capacity, capsid-modified hybrid adenovirus/adeno-associated virus vector for stable transduction of human hematopoietic cells. J Virol 2002; 76: 1135–1143.
Ueno T et al. Site-specific integration of a transgene mediated by a hybrid adenovirus/adeno-associated virus vector using the Cre/loxP-expression-switching system. Biochem Biophys Res Commun 2000; 273: 473–478.
Recchia A et al. Site-specific integration mediated by a hybrid adenovirus/adeno-associated virus vector. Proc Natl Acad Sci USA 1999; 96: 2615–2620.
Mizuguchi H et al. Tight positive regulation of transgene expression by a single adenovirus vector containing the rtTA and tTS expression cassettes in separate genome regions. Hum Gene Ther 2003; 14: 1265–1277.
Okada N et al. Efficient antigen gene transduction using Arg–Gly–Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res 2001; 61: 7913–7919.
Acknowledgements
We thank Tomomi Sasaki and Takashi Fukushima for technical assistance. We would also like to thank Dr J Miyazaki and Dr RG Hawley for kindly providing the CA promoter and the MSCV promoter, respectively. This work was supported in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by grants for Health and Labour Sciences Research from the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakurai, F., Kawabata, K., Yamaguchi, T. et al. Optimization of adenovirus serotype 35 vectors for efficient transduction in human hematopoietic progenitors: comparison of promoter activities. Gene Ther 12, 1424–1433 (2005). https://doi.org/10.1038/sj.gt.3302562
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302562
Keywords
This article is cited by
-
Heterologous expression of high-activity cytochrome P450 in mammalian cells
Scientific Reports (2020)
-
Efficient gene transfer into T lymphocytes by fiber-modified human adenovirus 5
BMC Biotechnology (2019)
-
Efficient detection of human circulating tumor cells without significant production of false-positive cells by a novel conditionally replicating adenovirus
Molecular Therapy - Methods & Clinical Development (2016)
-
NF-κB promotes leaky expression of adenovirus genes in a replication-incompetent adenovirus vector
Scientific Reports (2016)
-
Efficient antitumor effects of carrier cells loaded with a fiber-substituted conditionally replicating adenovirus on CAR-negative tumor cells
Cancer Gene Therapy (2012)