Abstract
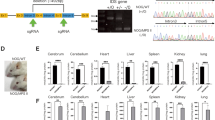
Current therapies for lysosomal storage diseases (LSDs), enzyme replacement therapy and bone marrow transplantation are effective for visceral organ pathology of LSD, but their effectiveness for brain involvement in LSDs is still a subject of controversy. As an alternative approach, we transplanted genetically modified bone marrow stromal (BMS) cells to lateral ventricle of newborn mucopolysaccharidosis VII (MPS VII) mice. MPS VII is one of LSDs and caused by deficiency of beta-glucuronidase (GUSB), resulting in accumulation of glycosaminoglycans (GAGs) in brain. At 2 weeks after transplantation, the GUSB enzyme-positive cells were identified in olfactory bulb, striatum and cerebral cortex, and the enzymatic activities in various brain areas increased. The GAGs contents in brain were reduced to near normal level at 4 weeks after transplantation. Although GUSB activity declined to homozygous level after 8 weeks, the reduction of GAGs persisted for 16 weeks. Microscopic examination indicated that the lysosomal distention was not found in treated animal brain. Cognitive function in MPS VII animals as evaluated by Morris Water Maze test in treated mice showed a marked improvement over nontreated animals. Brain transplantation of genetically modified BMS cells appears to be a promising approach to treat diffuse CNS involvement of LSDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Neufeld EF, Muenzer J . The mucopolysaccharidosis. In: Scriver CR (ed). In the Metabolic Basis of Inherited Disease. McGraw-Hill Inc: New York, 1989, pp 3421–3452.
Sly WS et al. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr 1973; 82: 249–257.
Birkenmeier EH et al. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest 1989; 83: 1258–1266.
Sands MS, Birkenmeier EH . A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci USA 1993; 90: 6567–6571.
Vogler C et al. A murine model of mucopolysaccharidosis VII. Gross and microscopic findings in beta-glucuronidase-deficient mice. Am J Pathol 1990; 136: 207–217.
Vogler C et al. Enzyme replacement with recombinant beta-glucuronidase in the newborn mucopolysaccharidosis type VII mouse. Pediatr Res 1993; 34: 837–840.
O’Connor LH et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII leads to improvements in behavior and auditory function. J Clin Invest 1998; 101: 1394–1400.
Sands MS et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest 1994; 93: 2324–2331.
Bastedo L et al. Behavioral consequences of bone marrow transplantation in the treatment of murine mucopolysaccharidosis type VII. J Clin Invest 1994; 94: 1180–1186.
Sands MS et al. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest 1993; 68: 676–686.
Birkenmeier EH et al. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood 1991; 78: 3081–3092.
Frisella WA et al. Intracranial injection of recombinant adeno-associated virus improves cognitive function in a murine model of mucopolysaccharidosis type VII. Mol Ther 2001; 3: 351–358.
Passini MA et al. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol 2003; 77: 7034–7040.
Brooks AI et al. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc Natl Acad Sci USA 2002; 99: 6216–6221.
Snyder EY, Taylor RM, Wolfe JH . Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature 1995; 374: 367–370.
Meng XL et al. Brain transplantation of genetically engineered human neural stem cells globally corrects brain lesions in the mucopolysaccharidosis type VII mouse. J Neurosci Res 2003; 74: 266–277.
Kosuga M et al. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol Ther 2001; 3: 139–148.
Pittenger MF et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Jiang Y et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418: 41–49.
Kohyama J et al. Brain from bone: efficient ‘meta-differentiation’ of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation 2001; 68: 235–244.
Kopen GC, Prockop DJ, Phinney DG . Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999; 96: 10711–10716.
Azizi SA et al. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc Natl Acad Sci USA 1998; 95: 3908–3913.
Zhao LR et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 2002; 174: 11–20.
Jin HK et al. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest 2002; 109: 1183–1191.
Taylor RM, Wolfe JH . Cross-correction of beta-glucuronidase deficiency by retroviral vector-mediated gene transfer. Exp Cell Res 1994; 214: 606–613.
Wolfe JH, Sands MS . Murine mucopoluysaccharidosis type VII: a model system for somatic gene transfer of the central nervous system. In: Lowenstein PR (ed). Protocols for Gene Transfer in Neuroscience: Towards Gene Therapy of Neurologic Disorders. John Wiley & Sons: New York, 1996, pp 263–274.
Halene S et al. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood 1999; 94: 3349–3357.
Shen JS et al. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther 2001; 8: 1081–1087.
Glaser JH, Sly WS . Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med 1973; 82: 969–977.
Shapira E et al. Biochemical Genetics: A Laboratory Manual. Oxford University press: New York, Oxford, 1989, pp 24–35.
Yoshida K et al. Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal Biochem 1989; 177: 327–332.
Morris R . Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47–60.
Chang PL, Lambert DT, Pisa MA . Behavioural abnormalities in a murine model of a human lysosomal storage disease. Neuroreport 1993; 4: 507–510.
Acknowledgements
We thank Drs DB Kohn (University of Southern California) and WS Sly (Saint Louis University School of Medicine) for kindly providing retrovirus vector and GUSB cDNA, respectively. We thank M Aoki and M Morimatsu of Laboratory Animal Center, The Jikei University School of Medicine, and H Maeda and H Fujita of Seikagaku Corporation for technical assistance. We also thank Dr SU Kim (University of British Columbia) for critical reviewing of this manuscript. This research was supported by the Bio-Venture Research Fund Project grant from the Ministry of Education, Science and Culture of Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakurai, K., Iizuka, S., Shen, JS. et al. Brain transplantation of genetically modified bone marrow stromal cells corrects CNS pathology and cognitive function in MPS VII mice. Gene Ther 11, 1475–1481 (2004). https://doi.org/10.1038/sj.gt.3302338
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302338