Abstract
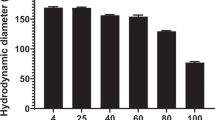
Dextran–spermine cationic polysaccharide was prepared by means of reductive amination between oxidized dextran and the natural oligoamine spermine. The formed Schiff-base imine-based conjugate was reduced with borohydride to obtain the stable amine-based conjugate. The transfection efficiency of the synthetic dextran–spermine was assessed in vitro on HEK293 and NIH3T3 cell lines and found to be as high as the DOTAP/Chol 1/1 lipid-based transfection reagent. Modification of the dextran–spermine polycation with polyethylene glycol resulted in high transfection yield in serum-rich medium. Intramuscular injection in mice of dextran–spermine–pSV-LacZ complex induced high local gene expression compared to low expression of the naked DNA. Intravenous injection of a dispersion of the dextran–spermine–pSV-LacZ complex resulted with no expression in all examined organs. When the partially PEGylated dextran–spermine–pSV-LacZ complex was intravenously applied, a high gene expression was detected mainly in the liver. Preliminary targeting studies indicated that the PEGylated dextran–spermine–pSV-LacZ complex bound to galactose receptor of liver parenchymal cells rather than the mannose receptor of liver nonparenchymal cells. This work offers a new biodegradable polycation based on natural components, which is capable of transfecting cells and tissues in vitro and in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schatzlein AG . Non-viral vectors in cancer gene therapy: principles and progress. Anti-Cancer Drugs 2001; 12: 275–304.
Mah C, Byrne BJ, Flotte TR . Virus-based gene delivery systems. Clin Pharmacokinet 2002; 41: 901–911.
Davis ME . Non-viral gene delivery systems. Curr Opin Biotechnol 2002; 13: 128–131.
Wiethoff CM, Middaugh CR . Barriers to nonviral gene delivery. J Pharm Sci 2003; 92: 203–217.
Schmidt-Wolf GD, Schmidt-Wolf IGH . Non-viral and hybrid vectors in human gene therapy: an update. Trends Mol Med 2003; 9: 67–72.
Putnam D et al. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc Natl Acad Sci USA 2001; 98: 1200–1205.
Kabanov AV . Taking polycation gene delivery systems from in vitro to in vivo. Pharm Sci Technol Today 1999; 2: 365–372.
Haynes M, Garrett RA, Gratzer WB . Structure of nucleic acid–poly base complexes. Biochemistry 1970; 9: 4410–4416.
De Smedt SC, Demeester J, Hennink WE . Cationic polymer based gene delivery systems. Pharm Res 2000; 17: 113–126.
Ferrari S et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Therapy 1997; 4: 1100–1106.
Abdallah B et al. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther 1996; 7: 1947–1954.
Vanderkerken S et al. Synthesis and evaluation of poly(ethylene glycol)–polylysine block copolymers as carriers for gene delivery. J Bioactive Compatible Polym 2000; 15: 115–138.
van de Wetering P et al. 2-(Dimethylamino)ethyl methacrylate based (co)polymers as gene transfer agents. J Control Release 1998; 53: 145–153.
Wolfert MA et al. Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block co-polymers. Hum Gene Ther 1996; 7: 2123–2133.
Yaroslavov AA et al. DNA affinity to biological membranes is enhanced due to complexation with hydrophobized polycation. FEBS Lett 1996; 384: 177–180.
MacLaughlin FC et al. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Control Release 1998; 56: 259–272.
Takai T, Ohmori H . DNA transfection of mouse lymphoid-cells by the combination of DEAE-dextran-mediated DNA uptake and osmotic shock procedure. Biochim Biophys Acta 1990; 1048: 105–109.
Behr JP . The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 1997; 51: 34–36.
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo – polyethylenimine. Proc Natl Acad Sci USA 1995; 92: 7297–7301.
Mahato RI, Takakura Y, Hashida M . Nonviral vectors for in vivo gene delivery: physicochemical and pharmacokinetic considerations. Crit Rev Therap Drug Carrier Systems 1997; 14: 133–172.
Tomlinson E, Rolland AP . Controllable gene therapy – pharmaceutics of non-viral gene delivery systems. J Control Release 1996; 39: 357–372.
Pouton CW, Seymour LW . Key issues in non-viral gene delivery. Adv Drug Deliv Rev 1998; 34: 3–19.
Kawabata K, Takakura Y, Hashida M . The fate of plasmid DNA after intravenous-injection in mice – involvement of scavenger receptors in its hepatic-uptake. Pharm Res 1995; 12: 825–830.
Yu L et al. Systemic administration of Terplex DNA system: pharmacokinetics and gene expression. Pharm Res 2001; 18: 1277–1283.
Kircheis R et al. Tumor targeting with surface-shielded ligand–polycation DNA complexes. J Control Release 2001; 72: 165–170.
Liu F, Huang L . Development of non-viral vectors for systemic gene delivery. J Control Release 2002; 78: 259–266.
Templeton NS et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 1997; 15: 647–652.
Torchilin VP et al. Amphiphilic vinyl-polymers effectively prolong liposome circulation time in vivo. Biochim Biophys Acta – Biomembr 1994; 1195: 181–184.
Woodle MC, Engbers CM, Zalipsky S . New amphipathic polymer lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjugate Chem 1994; 5: 493–496.
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol 1997; 15: 167–173.
Goula D et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Therapy 1998; 5: 1291–1295.
Hofland HEJ et al. In vivo gene transfer by intravenous administration of stable cationic lipid DNA complex. Pharm Res 1997; 14: 742–749.
Mahato RI et al. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration. Hum Gene Ther 1998; 9: 2083–2099.
Azzam T et al. Polysaccharide–oligoamine based conjugates for gene delivery. J Med Chem 2002; 45: 1817–1824.
Azzam T et al. Cationic polysaccharides for gene delivery. Macromolecules 2002; 35: 9947–9953.
Azzam T et al. Dextra-Spermine conjugate: An Efficient Vector for Gene Delivery. Macromol Symp 2003; 195: 247–261.
Ogris M et al. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy 1999; 6: 595–605.
Pepinsky RB et al. Long-acting forms of sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are efficacious in a nerve injury model. J Pharm Sci 2002; 91: 371–387.
Lasic DD . Recent developments in medical applications of liposomes: sterically stabilized liposomes in cancer therapy and gene delivery in vivo. Control Release 1997; 48: 203–222.
Kaneo Y et al. Pharmacokinetics and biodisposition of fluorescein-labeled arabinogalactan in rats. Int J Pharm 2000; 201: 59–69.
Hashida M et al. Targeted delivery of plasmid DNA complexed with galactosylated poly(L-lysine). J Control Release 1998; 53: 301–310.
Ogawara KI et al. Pharmacokinetic evaluation of mannosylated bovine serum albumin as a liver cell-specific carrier: quantitative comparison with other hepatotropic ligands. J Drug Target 1999; 6: 349–360.
Zhao H, Heindel ND . Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm Res 1991; 8: 400–402.
Kumar N, Azzam T, Domb AJ . Molecular mass distribution of polycations and dextrans by high-performance size exclusion chromatography. Polym Adv Technol 2002; 13: 1071–1077.
Snyder SL, Sobocinski PZ . An improved 2,4,6-trinitro- benzenesulfonic acid method for the determination of amines. Anal Biochem 1975; 64: 284–288.
Acknowledgements
Tony Azzam is grateful to the Ministry of Science, Israel, for its generous financial assistance. This work was supported in part by the AFIRST, French–Israeli Cooperation on Gene Therapy, and by the US–Israel Binational Fund (BSF).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hosseinkhani, H., Azzam, T., Tabata, Y. et al. Dextran–spermine polycation: an efficient nonviral vector for in vitro and in vivo gene transfection. Gene Ther 11, 194–203 (2004). https://doi.org/10.1038/sj.gt.3302159
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302159
Keywords
This article is cited by
-
Diseases originate and terminate by genes: unraveling nonviral gene delivery
Drug Delivery and Translational Research (2013)
-
Synthesis and aminolysis of polysaccharide carbonates
Cellulose (2013)
-
Rapid bioreduction of trivalent aurum using banana stem powder and its cytotoxicity against MCF-7 and HEK-293 cell lines
Journal of Nanoparticle Research (2013)
-
Co-transfection Gene Delivery of Dendritic Cells Induced Effective Lymph Node Targeting and Anti-tumor Vaccination
Pharmaceutical Research (2013)
-
Biodegradable nanoparticles for gene therapy technology
Journal of Nanoparticle Research (2013)