Abstract
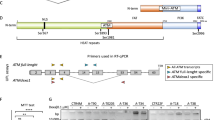
Ataxia-telangiectasia (AT) is a human autosomal recessive disease with a pleiotropic phenotype characterized by cerebellar degeneration, immunodeficiency, premature aging, cancer predisposition, and radiation sensitivity. The gene mutated in AT, ATM (for AT-mutated), had been cloned and found to have ionizing radiation and oxidative stress-inducible kinase activity. No treatment can stop the progression of the disease. In this study, the complete open-reading frame of ATM cDNA was cloned into a Herpes simplex virus type-1 (HSV-1) amplicon vector (pTO-ATM), and the transduction of cultured AT cells was demonstrated by immunohistochemistry and Western blot analysis. Functional gene expression was evaluated by cell colony-forming assays following exposure to oxidative stress. The survival of AT cells with ATM gene transduction was about 100% higher compared to nontransduced cells after t-butyl hydroperoxide treatments. Next, the normal ATM gene expression in different regions of the rat brain was studied. Immunohistochemistry staining demonstrated weak endogenous ATM protein expression in neurons of the caudate-putamen, with significantly higher levels of expression detected in neurons in other brain regions. Exogenous ATM gene expression from pTO-ATM after viral transduction in the caudate-putamen of the adult rat was examined. At 3 days after injection of the pTO-ATM viral vector, abundant positive ATM staining of the neurons was found at the injection sites, in comparison to the controls. These data demonstrate that the relatively large ATM cDNA can be transduced and expressed in vitro and in vivo from an HSV amplicon viral vector. These data provide initial evidence that the replacement of the ATM gene into the cells of AT patients might be possible some day.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boder E . Ataxia-telangiectasia: an overview. Kroc Found Ser 1985; 19: 1–63.
McKinnon PJ . Ataxia-telangiectasia: an inherited disorder of ionizing-radiation sensitivity in man. Progress in the elucidation of the underlying biochemical defect. Hum Genet 1987; 75: 197–208.
Sedgwick R, Boder E . Ataxia-telangiectasia. Elsevier: Amsterdam, 1991.
Vinters HV, Gatti RA, Rakic P . Sequence of cellular events in cerebellar ontogeny relevant to expression of neuronal abnormalities in ataxia-telangiectasia. Kroc Found Ser 1985; 19: 233–255.
Savitsky K et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase (see comments). Science 1995; 268: 1749–1753.
Savitsky K et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet 1995; 4: 2025–2032.
Watters D et al. Cellular localisation of the ataxia-telangiectasia (ATM) gene product and discrimination between mutated and normal forms. Oncogene 1997; 14: 1911–1921.
Barlow C et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci USA 2000; 97: 871–876.
Jackson SP . Cancer predisposition. Ataxia-telangiectasia at the crossroads. Curr Biol 1995; 5: 1210–1212.
Lavin MF et al. Relationship of the ataxia-telangiectasia protein ATM to phosphoinositide 3-kinase. Trends Biochem Sci 1995; 20: 382–383.
Lehmann AR, Carr AM . The ataxia-telangiectasia gene: a link between checkpoint controls, neurodegeneration and cancer. Trends Genet 1995; 11: 375–377.
Zakian VA . ATM-related genes: what do they tell us about functions of the human gene? Cell 1995; 82: 685–687.
Rotman G, Shiloh Y . ATM: from gene to function. Hum Mol Genet 1998; 7: 1555–1563.
Taylor AM . What has the cloning of the ATM gene told us about ataxia telangiectasia? Int J Radiat Biol 1998; 73: 365–371.
Shiloh Y, Kastan MB . ATM: genome stability, neuronal development, and cancer cross paths. Adv Cancer Res 2001; 83: 209–254.
Shiloh Y . ATM (ataxia telangiectasia mutated): expanding roles in the DNA damage response and cellular homeostasis. Biochem Soc Trans 2001; 29: 661–666.
Barzilai A, Rotman G, Shiloh Y . ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002; 1: 3–25.
Chiocca EA et al. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol 1990; 2: 739–746.
During MJ, Naegele JR, O'Malley KL, Geller AI . Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase (see comments). Science 1994; 266: 1399–1403.
Bennett JJ et al. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. J Mol Med 2000; 78: 166–174.
Natsume A et al. Bcl-2 and GDNF delivered by HSV-mediated gene transfer act additively to protect dopaminergic neurons from 6-OHDA-induced degeneration. Exp Neurol 2001; 169: 231–238.
Yenari MA, Dumas TC, Sapolsky RM, Steinberg GK . Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Ann N Y Acad Sci 2001; 939: 340–357.
Poliani PL et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther 2001; 12: 905–920.
Markert JM et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy 2000; 7: 867–874.
Rampling R et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy 2000; 7: 859–866.
Papanastassiou V et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoral injection into human malignant glioma: a proof of principle study. Gene Therapy 2002; 9: 398–406.
Spaete RR, Frenkel N . The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell 1982; 30: 295–304.
Geller AI, Breakefield XO . A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science 1988; 241: 1667–1669.
Geller AI, Freese A . Infection of cultured central nervous system neurons with a defective herpes simplex virus 1 vector results in stable expression of Escherichia coli beta-galactosidase. Proc Natl Acad Sci USA 1990; 87: 1149–1153.
Kwong AD, Frenkel N . The herpes simplex virus amplicon. IV. Efficient expression of a chimeric chicken ovalbumin gene amplified within defective virus genomes. Virology 1985; 142: 421–425.
Carew JF et al. Efficient gene transfer to human squamous cell carcinomas by the herpes simplex virus type 1 amplicon vector. Am J Surg 1998; 176: 404–408.
Levatte MA, Cassam AK, Dekaban GA, Weaver LC . Analysis of a multi-mutant herpes simplex virus type 1 for gene transfer into sympathetic preganglionic neurons and a comparison to adenovirus vectors. Neuroscience 1998; 86: 1321–1336.
Wang S et al. A novel herpes virus amplicon system for in vivo gene delivery. Gene Therapy 1997; 4: 1132–1141.
Wang S, Vos J . A hybrid herpes virus infectious vector based on Epstein–Barr virus and Herpes Simplex Virus type 1 for gene transfer into human cells in vitro and in vivo. J Virol 1996; 70: 8422–8430.
Yamada M et al. Herpes simplex virus vector-mediated expression of Bcl-2 prevents 6-hydroxydopamine-induced degeneration of neurons in the substantia nigra in vivo. Proc Natl Acad Sci USA 1999; 96: 4078–4083.
Strathdee CA, McLeod MR . A modular set of helper-dependent herpes simplex virus expression vectors. Mol Ther 2000; 1: 479–485.
Simonato M, Manservigi R, Marconi P, Glorioso J . Gene transfer into neurones for the molecular analysis of behaviour: focus on herpes simplex vectors. Trends Neurosci 2000; 23: 183–190.
Agudo M et al. Highly efficient and specific gene transfer to Purkinje cells in vivo using a herpes simplex virus I amplicon. Hum Gene Ther 2002; 13: 665–674.
Uziel T et al. Genomic organization of the ATM gene. Genomics 1996; 33: 317–320.
Zhang N et al. Isolation of full-length ATM cDNA and correction of the ataxia-telangiectasia cellular phenotype. Proc Natl Acad Sci USA 1997; 94: 8021–8026.
Ziv Y et al. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 1997; 15: 159–167.
Scott SP et al. Cloning and expression of the ataxia-telangiectasia gene in baculovirus. Biochem Biophys Res Commun 1998; 245: 144–148.
Ho DY, Mocarski ES, Sapolsky RM . Altering central nervous system physiology with a defective herpes simplex virus vector expressing the glucose transporter gene. Proc Natl Acad Sci USA 1993; 90: 3655–3659.
D'Angelica M et al. In vivo interleukin-2 gene therapy of established tumors with herpes simplex amplicon vectors. Cancer Immunol Immunother 1999; 47: 265–271.
Marconi P et al. Replication-defective herpes simplex virus vectors for neurotrophic factor gene transfer in vitro and in vivo. Gene Therapy 1999; 6: 904–912.
Gatti RA, Vinters HV . Cerebellar pathology in ataxia-telangiectasia: the significance of basket cells. Kroc Found Ser 1985; 19: 225–232.
Lakin ND et al. Analysis of the ATM protein in wild-type and ataxia telangiectasia cells. Oncogene 1996; 13: 2707–2716.
Soares HD, Morgan JI, McKinnon PJ . Atm expression patterns suggest a contribution from the peripheral nervous system to the phenotype of ataxia-telangiectasia. Neuroscience 1998; 86: 1045–1054.
Oka A, Takashima S . Expression of the ataxia-telangiectasia gene (ATM) product in human cerebellar neurons during development. Neurosci Lett 1998; 252: 195–198.
Johnson PA et al. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol 1992; 66: 2952–2965.
Sabel BA, Vick A, Holt V . Neurotoxic reactions of CNS following gene transfer with defective herpes simplex virus (HSV-1) vector. NeuroReport 1995; 6: 2447–2449.
Glorioso JC et al. HSV as a gene transfer vector for the nervous system. Mol Biotechnol 1995; 4: 87–99.
Wu N, Watkins SC, Schaffer PA, DeLuca NA . Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol 1996; 70: 6358–6369.
Fink DJ, Glorioso JC . Engineering herpes simplex virus vectors for gene transfer to neurons. Nat Med 1997; 3: 357–359.
Samaniego LA, Wu N, DeLuca NA . The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol 1997; 71: 4614–4625.
Krisky DM et al. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Therapy 1998; 5: 1593–1603.
Lilley CE et al. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J Virol 2001; 75: 4343–4356.
Burton EA, Bai Q, Goins WF, Glorioso JC . Replication-defective genomic herpes simplex vectors: design and production. Curr Opin Biotechnol 2002; 13: 424–428.
Fraefel C et al. Helper virus-free transfer of Herpes simplex virus type 1 plasmid vectors into neural cells. J Virol 1996; 70: 7190–7197.
Stavropoulos TA, Strathdee CA . An enhanced packaging system for helper-dependent herpes simplex virus vectors. J Virol 1998; 72: 7137–7143.
Saeki Y et al. Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol Ther 2001; 3: 591–601.
Logvinoff C, Epstein AL . A novel approach for herpes simplex virus type 1 amplicon vector production, using the Cre-loxP recombination system to remove helper virus. Hum Gene Ther 2001; 12: 161–167.
Olschowka JA et al. Helper-free HSV-1 amplicons elicit a markedly less robust innate immune response in the CNS. Mol Ther 2003; 7: 218–227.
Smith IL, Hardwicke MA, Sandri-Goldin RM . Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 1992; 186: 74–86.
McCarthy AM, McMahan L, Schaffer PA . Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol 1989; 63: 18–27.
Shackelford RE et al. The ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts. J Biol Chem 2001; 276: 21951–21959.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, 1986.
Acknowledgements
We are grateful to Dr N Stow, Dr D Cortez, Dr RS Paules, and Dr Y Shiloh for the generous gifts of materials. We would like to thank Jian Wang, Jun Guo, and Mathew Lux for technical assistance. We also thank Dr Yosif Shiloh for his help with manuscript preparation. This study was partly supported by the AT Children's Project, FL, USA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qi, J., Shackelford, R., Manuszak, R. et al. Functional expression of ATM gene carried by HSV amplicon vector in vitro and in vivo. Gene Ther 11, 25–33 (2004). https://doi.org/10.1038/sj.gt.3302140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302140
Keywords
This article is cited by
-
NF1 patient missense variants predict a role for ATM in modifying neurofibroma initiation
Acta Neuropathologica (2020)
-
Targeted Integration of Functional Human ATM cDNA Into Genome Mediated by HSV/AAV Hybrid Amplicon Vector
Molecular Therapy (2008)