Abstract
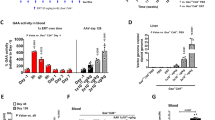
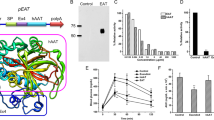
Therapy in glycogen storage disease type Ia (GSD Ia), an inherited disorder of carbohydrate metabolism, relies on nutritional support that postpones but fails to prevent long-term complications of GSD Ia. In the canine model for GSD Ia, we evaluated the potential of intravenously delivered adeno-associated virus (AAV) vectors for gene therapy. In three affected canines, liver glycogen was reduced following hepatic expression of canine glucose-6-phosphatase (G6Pase). Two months after AAV vector administration, one affected dog had normalization of fasting glucose, cholesterol, triglycerides, and lactic acid. Concatamerized AAV vector DNA was confirmed by Southern blot analysis of liver DNA isolated from treated dogs, as head-to-tail, head-to-head, and tail-to-tail concatamers. Six weeks after vector administration, the level of vector DNA signal in each dog varied from one to five copies per cell, consistent with variation in the efficiency of transduction within the liver. AAV vector administration in the canine model for GSD Ia resulted in sustained G6Pase expression and improvement in liver histology and in biochemical parameters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koeberl DD et al. Persistent expression of human clotting factor IX form mouse liver after intravenous injection of adeno-associated virus vectors Proc Natl Acad Sci USA 1997 94: 1426–1431
Snyder RO et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors Nat Med 1999 5: 64–70
Snyder RO et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors Nat Genet 1997 16: 270–276
Nakai H et al. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver Blood 1998 91: 4600–4607
Wang L et al. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy Proc Natl Acad Sci USA 1999 96: 3906–3910
Kessler PD et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein Proc Natl Acad Sci USA 1996 93: 14082–14087
Xiao W et al. Adeno-associated virus as a vector for liver-directed gene therapy J Virol 1998 72: 10222–10226
Fisher KJ et al. Recombinant adeno-associated virus for muscle directed gene therapy Nat Med 1997 3: 306–312
Herzog RW et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus Proc Natl Acad Sci USA 1997 94: 5804–5809
Herzog RW et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector Nat Med 1999 5: 56–62
Kay MA et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector Nat Genet 2000 24: 257–261
von Gierke E . Hepato-nephro-megalia glycogenica (Glykogenspeicher-krankheit der Leber und Nieren) Beitr Pathol Anat 1929 82: 497
Cori GT, Cori CF . Glucose-6-phosphatase of the liver in glycogen stoarage disease J Biol Chem 1952 199: 661–667
Chen YT . Glycogen storage diseases Scriver CR, Beaudet AL, Sly WS, Valle D (eds); The Metabolic and Molecular Basis of Inherited Disease McGraw-Hill 2001 pp 1521–1551
Kishnani PS, Bengur AR, Chen YT . Pulmonary hypertension in glycogen storage disease type I J Inher Metab Dis 1996 19: 213–216
Ohura T et al. Progressive pulmonary hypertension: a fatal complication of type I glycogen storage disease J Inher Metab Dis 1995 18: 361
Talente GM et al. Glycogen storage disease in adults Ann Int Med 1994 120: 218–226
Chen YT et al. Type I glycogen storage disease: nine years of management with cornstarch Eur J Pediatr 1993 152: S56–S59
Wolfsdorf JI . Crigler Jr. JJ. Effect of continuous glucose therapy begin in infancy on the long-term clinical course of patients with type I glycogen storage disease J Ped Gastrol Nutr 1999 29: 136–143
Wolfsdorf JI . Crigler Jr. JJ. Biochemical evidence for the requirement of continuous glucose therapy in young adults with type I glycogen storage disease J Inher Metab Dis 1994 17: 234–241
Lee PJ et al. Glomerular and tubular function in glycogen storage disease Ped Nephrol 1995 9: 705–710
Wolfsdorf JI . Crigler Jr.. JJ. Metabolic control and renal dysfunction in type I glycogen storage disease J Inher Metab Dis 1997 20: 559–568
Brix AE et al. Glycogen storage disease type Ia in two littermate Maltese puppies Vet Pathol 1995 32: 460–465
Kishnani P et al. Isolation and nucleotide sequence of canine glucose-6-phospatase mRNA: identification of mutation in puppies with glycogen storage disease type Ia Biochem Mol Med 1997 61: 168–177
Kishnani P et al. Canine model and genomic structure organization of glycogen storage disease type Ia (GSD Ia) Vet Pathol 2001 38: 83–89
Matern D et al. Liver transplantation for glycogen storage disease types I, III and IV Eur J Ped 1997 158: S43–S48
Miao C et al. The kinetic of rAAV integration in the liver Nat Genet 1998 19: 13–15
Zingone A et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy J Biol Chem 2000 275: 828–832
Perlman M et al. Successful treatment of severe type I glycogen storage disease with neonatal presentation by nocturnal intragastric feeding J Ped 1979 94: 772–774
Miao CH et al. Nonrandom transduction of recombinant adeno-associated virus vectors in mouse hepatocytes in vivo: cell cycling does not influence hepatocyte transduction J Virol 2000 74: 3793–3803
Jung S-C et al. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice Proc Natl Acad Sci USA 2001 98: 2676–2681
Koeberl DD et al. Persistent, therapeutically relevant levels of human granulocyte colony-stimulating factor in mice after systemic delivery of adeno-associated virus vectors Hum Gene Ther 1999 10: 2133–2140
Nakai H et al. Identification of intermediates for recombinant adeno-associated virus vector genome concatemerization and integration in hepatocytes in vivo: evidence for double-stranded linear monomer genomes not circles as reactive intermediates Mol Ther 2001 3: S132
Pinkert CA et al. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice Genes Dev 1987 1: 268–276
Brinster RL et al. Introns increase transcriptional efficiency in transgenic mice Proc Natl Acad Sci USA 1988 85: 836–840
Allen JM, Halbert CL, Miller DM . Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6 Mol Ther 2000 1: 88–95
Amalfitano A et al. Production and characterization of improved adenovirus vectors with the E1, E2b and E3 genes deleted J Virol 1998 72: 926–933
Amalfitano A et al. Production and characterization of improved adenovirus vectors with the E1, E2b and E3 genes deleted Snyder RO et al. Vectors for gene therapy, unit 12.1: production of recombinant adeno-associated viral vectors. In: Dracopoli NC et al (eds). Current Protocols in Human Genetics. John Wiley: Chichester, 1996, pp 12.1.1–12.1.4.
Acknowledgements
We thank Ibrahim Bori, Eric Faulkner, Kathy Frid, Kwang Ok Shin and Joe Zeidner for excellent technical support, and Andrea Amalfitano and Dieksha Bali for insightful comments. We are very grateful to Dr Steve Van Camp for his contributions to the establishment of the canine GSD Ia colony. This work was supported a Howard Hughes Young Investigator Award (DDK), and by the Association for Glycogen Storage Disease.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beaty, R., Jackson, M., Peterson, D. et al. Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors. Gene Ther 9, 1015–1022 (2002). https://doi.org/10.1038/sj.gt.3301728
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301728
Keywords
This article is cited by
-
Studies on glycogen storage disease type 1a animal models: a brief perspective
Transgenic Research (2022)
-
Rescue administration of a helper-dependent adenovirus vector with long-term efficacy in dogs with glycogen storage disease type Ia
Gene Therapy (2012)
-
In search of proof‐of‐concept: gene therapy for glycogen storage disease type Ia
Journal of Inherited Metabolic Disease (2012)
-
Hepatorenal Correction in Murine Glycogen Storage Disease Type I With a Double-stranded Adeno-associated Virus Vector
Molecular Therapy (2011)
-
AAV2/8-mediated Correction of OTC Deficiency Is Robust in Adult but Not Neonatal Spfash Mice
Molecular Therapy (2009)