Abstract
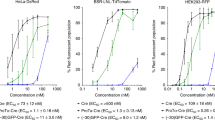
Recent studies have indicated that there are many barriers to successful systemic gene delivery via cationic lipid vectors using the intravenous route. The purpose of this study was to investigate the effect of binding and interaction between erythrocytes, a major constituent of blood cells, and the complexes, in relation to the role of the helper lipid, on the in vivo gene delivery to the lung following intravenous injection. We used three types of cationic lipid vectors, DNA–DOTMA/Chol liposome complexes, DNA–DOTMA liposome complexes, and DNA–DOTMA/DOPE liposome complexes. Although the three types of vectors bind to murine blood cells in vivo and in vitro, DOTMA/Chol and DOTMA complexes with a higher in vivo transfection activity do not induce fusion between erythrocytes, whereas DOTMA/DOPE complexes, a less efficient vector in vivo, induce fusion between the erythrocytes after a short incubation period. Pre-incubation of DOTMA/DOPE complexes with erythrocytes significantly reduced the transfection efficiency while DOTMA/Chol- and DOTMA complexes were more resistant to such treatment. The differences in the physicochemical and structural properties of these complexes could explain the differences in interaction with erythrocytes and subsequent gene expression. Lipids in DOTMA/Chol and DOTMA complexes have a stable lamellar structure. However, lipids in DOTMA/DOPE complexes have a highly curved structure with high fluidity. These results indicate that the interaction with erythrocytes depends on the properties of the cationic lipid vectors and this is an important factor for intravenous gene delivery using cationic lipid vectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Felgner JH et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations J Biol Chem 1994 269: 2550–2561
Wheeler CJ et al. A novel cationic lipid greatly enhances plasmid DNA delivery and expression in mouse lung Proc Natl Acad Sci USA 1996 93: 11454–11459
Caplen NJ et al. In vitro liposome-mediated DNA transfection of epithelial cell lines using the cationic liposome DC-Chol/DOPE Gene Therapy 1995 2: 603–613
Sternberg B, Hong K, Zheng W, Papahadjopoulos D . Ultrastructural characterization of cationic liposome–DNA complexes showing enhanced stability in serum and high transfection activity in vivo Biochim Biophys Acta 1998 1375: 23–35
Lee ER et al. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung Hum Gene Ther 1996 7: 1701–1717
Barron LG, Gagne L, Scoka FC Jr . Lipoplex-mediated gene delivery to the lung occurs within 60 minutes of intravenous administration Hum Gene Ther 1999 10: 1683–1694
Lasic DD . Structure and structure–activity relationships of lipid-based gene delivery systems. In: Huang L, Hung M-C, Wagner E. (eds) Nonviral Vectors for Gene Therapy Academic Press: San Diego 1999 69–87
Kikuchi A et al. Development of novel cationic liposomes for efficient gene transfer into peritoneal disseminated tumor Hum Gene Ther 1999 10: 947–955
Stephan DJ et al. A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo Hum Gene Ther 1996 7: 1803–1812
Dow SW et al. Systemic and local interferon γ gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice Hum Gene Ther 1999 10: 1905–1914
Nabel GJ et al. Direct gene transfer with DNA–liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans Proc Natl Acad Sci USA 1993 90: 11307–11311
Caplen NJ et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Nature Med 1995 1: 39–46
Farhood H, Serbina N, Huang L . The role of dioleoyl phophatidylethanolamine in cationic liposome mediated gene transfer Biochim Biophys Acta 1995 1235: 289–295
Horton HM et al. IL-2 plasmid therapy of murine ovarian carcinoma inhibits the growth of tumor ascites and alters its cytokine profile J Immunol 1999 163: 6378–6385
Koltover I, Salditt T, Radler O, Safinya CR . An inverted hexagonal phase of cationic liposome–DNA complexes related to DNA release and delivery Science 1998 281: 78–81
Noguchi A, Furuno T, Kawaura C, Nakanishi M . Membrane fusion plays an important role in gene transfection mediated by cationic liposomes FEBS Lett 1998 433: 169–173
Ouahabi AE et al. The role of endosome destabilizing activity in the gene transfer process mediated by cationic lipids FEBS Lett 1997 414: 187–192
Wrobel I, Collins D . Fusion of cationic liposomes with mammalian cells occurs after endocytosis Biochim Biophys Acta 1995 1235: 296–304
Templeton NS et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression Nature Biotechnol 1997 15: 647–652
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery Nat Biotechnol 1997 15: 167–173
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid-protamine–DNA (LPD) complexes for intravenous gene delivery Gene Therapy 1998 5: 930–937
Li S et al. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection Gene Therapy 1999 6: 585–594
Mclean JW, Thurstin G, McDonald DM . Site of uptake and expression of cationic liposome/DNA complexes injected intravenously. In: Huang L, Hung M-C, Wagner E (eds) Nonviral Vectors for Gene Therapy Academic Press: San Diego 1999 120–134
McLean JW et al. Organ-specific endothelial cell uptake of cationic liposome–DNA complexes in mice Am J Physiol Heart Circ Physiol 1997 42: H384–H404
Felgner PL et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure Proc Natl Acad Sci USA 1987 84: 7413–7414
Song YK, Liu F, Chu S, Liu D . Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration Gene Therapy 1997 8: 1585–1594
Eastman SJ et al. Biophysical characterization of cationic lipid:DNA complexes Biochim Biophys Acta 1997 1325: 41–62
Song YK, Liu D . Free liposomes enhance the transfection activity of DNA/lipid complexes in vivo by intravenous administration Biochim Biophys Acta 1998 1372: 141–150
Boukhnikachvili T et al. Structure of in-serum transfection DNA–cationic lipid complexes FEBS Lett 1997 409: 188–194
Sternberg B, Sorgi FL, Huang L . New structures in complex formation between DNA and cationic liposomes visualized by freeze-fracture electron microscopy FEBS Lett 1994 356: 361–366
Talbot WA, Zheng LX, Lentz BR . Acyl chain unsaturation and vesicle curvature alter outer leaflet packing and promote poly(ethylene glycol)-mediated membrane fusion Biochemistry 1997 36: 5827–5836
Sieger DP et al. Physiological levels of diacylglycerols in phospholipid membranes induced membrane fusion and stabilize inverted phases Biochemistry 1989 28: 3703–3709
Sakurai F et al. Effects of blood components on lung accumulation of lipoplexes in the single-pass rat lung perfusion system Eur J Pharm Biopharm (submitted)
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes Gene Therapy 1997 4: 891–900
Shinitzky M, Barenholz Y . Fluidity parameters of lipid regions determined by fluorescence polarization Biochim Biophys Acta 1978 515: 367–394
Takino T et al. Pharmacokinetic disposition analysis of lipophilic drugs injected with various lipid carriers in the single-pass rat liver perfusion system Int J Pharm 1995 114: 43–54
Senior JH, Trimble KR, Maskiewicz R . Interaction of positively charged liposomes with blood: implication for their application in vivo Biochim Biophys Acta 1991 1070: 173–179
Fabry ME et al. High expression of human βs- and α-globins in transgenic mice: hemoglobin composition and hematological consequences Proc Natl Acad Sci USA 1992 89: 12150–12154
Acknowledgements
This work was supported in part by a Grant-In-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakurai, F., Nishioka, T., Saito, H. et al. Interaction between DNA–cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: the role of the neutral helper lipid. Gene Ther 8, 677–686 (2001). https://doi.org/10.1038/sj.gt.3301460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301460
Keywords
This article is cited by
-
Polyprenol-Based Lipofecting Agents for In Vivo Delivery of Therapeutic DNA to Treat Hypertensive Rats
Biochemical Genetics (2021)
-
Recent advancements in the use of exosomes as drug delivery systems
Journal of Nanobiotechnology (2018)
-
Assessing the effect of a nude mouse model on nanoparticle-mediated gene delivery
Drug Delivery and Translational Research (2017)
-
Systemic delivery of E6/7 siRNA using novel lipidic particles and its application with cisplatin in cervical cancer mouse models
Gene Therapy (2011)
-
Positive Correlation Between the Generation of Reactive Oxygen Species and Activation/Reactivation of Transgene Expression After Hydrodynamic Injections into Mice
Pharmaceutical Research (2011)