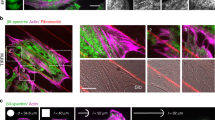
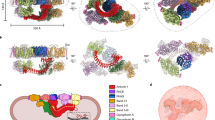
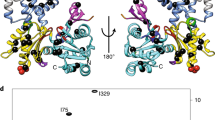
Abstract
The spectrin-based membrane skeleton, an assembly of proteins tightly associated with the plasma membrane, determines the shape and mechanical properties of erythrocytes. Spectrin, the most abundant component of this assembly, is an elongated and flexible molecule that, with potentiation by protein 4.1, is cross-linked at its ends by short actin filaments to form a lattice beneath the membrane. These and other proteins stabilize the plasma membrane, organize integral membrane proteins and maintain specialized regions of the cell surface1,3. A membrane-skeleton-associated calmodulin-binding protein of erythrocytes4 is a major substrate for Ca2+- and phospholipid-dependent protein kinase C (ref. 5), and thus is a target for Ca2+ by two regulatory pathways. Here we demonstrate that this protein, called adducin: (1) binds tightly in vitro to spectrin-actin complexes but with much less affinity either to spectrin or to actin alone; (2) promotes assembly of additional spectrin molecules onto actin filaments; and (3) is inhibited in its ability to induce the binding of additional spectrin molecules to actin by micromolar concentrations of calmodulin and Ca2+. Adducin may be involved in the action of Ca2+ on erythrocyte membrane skeleton and in the assembly of spectrin-actin complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Marchesi, V. T. A. Rev. Cell Biol. 1, 531–561 (1985). 2. Bennett, V. A. Rev. Biochem. 54, 273–304 (1985). 3. Goodman, S. R. and Zagon, I. S. Am. J. Physiol. 250, 347–360 (1986). 4. Gardner, K. & Bennett, V. J. biol. Chem. 260, 1339–1348 (1986). 5. Ling, E., Gardner, K. & Bennett, V. /. biol. Chem. 261, 13875–13878 (1986). 6. Brenner, S. & Korn, E. / biol. Chem. 254, 8620–8627 (1979). 7. Bennett, V., Davis J. & Fowler, V. E. Nature 299, 126–131 (1982). 8. Gardner, K. & Bennett, V. J. cell biochem. (in the press). 9. Cheung, W. Y. Science 207, 19–27 (1980). 10. Burns, N. & Gratzer, W. Biochemistry 24, 3070–3074 (1985). 11. Husain, A., Howelett, G. J. & Sawyer, W. H. Biochem. biophys. Res. Commun. 122,1194–1200 (1984). 12. Sears, D. E., Marchesi, V. T. & Morrow, J. S. Biochem. biophys. Acta 870, 432–442 (1986). 13. Agre, P., Gardner, K. & Bennett, V. / biol. Chem. 258, 6258–6265 (1983). 14. Jarrett, H. & Penniston J T. J. biol. Chem. 253, 4676–4682 (1978). 15. Ohanian, V. et al. Biochemistry 23, 4416–4420 (1984). 16. Ungewickell, E., Bennett, P., Calvert, R., Ohanion, V. & Gratzer, W. Nature 280, 811–814 (1979). 17. Tyler, J. M, Reinardt, B. N. & Branton, D. / biol. Chem. 255, 7034–7039 (1980). 18. Cohen, C. M. & Foley, S. F. Biochemistry 23, 6091–6098 (1984). 19. Eder, P. S., Soong, C. J. & Tao, M. Biochemistry 25, 1764–1770 (1986). 20. Staufenbiel, M. & Lazarides, E. / Cell Biol. 102, 1157–1163 (1986). 21. Conboy, J., Mohandas, N., Tchernia, G. & Kan, Y. W. N. Engl. J. Med. 315,680–685 (1986). 22. Beaven, G. H. et al. Eur. J. Cell Biol. 36, 299–306 (1985). 23. Byers, T. J. & Branton, D. Proc. natn. Acad. Sci. U.S.A. 82, 6153–6157 (1985). 24. Shen, B. W., Josephs, R. & Steck, T. L. J. Cell Biol. 102, 997–1006 (1986). 25. Liu, S.–C., Derick, L. H. & Palek, J. J. Cell Biol. 104, 527–536 (1986). 26. Takakuwa, Y., Tchernia, G., Rossi, M., Benabadji, M. & Mohandas, N. / din. Invest. 78, 80–85 (1986). 27. Bourguignon, L. Y., Suchard, S. J., Nagpal, M. L. & Glenney, J. R. Jr J. Cell Biol. 101, 477–487 (1985). 28. Perrin, D. & Aunis, D. Nature 318, 585–591 (1985). 29. Fowler, V. M. & Bennett, V. /. biol. Chem. 259, 5978–5989 (1982). 30. Pardee, J. D. & Spudich, J. A. Meth. Cell Biol. 24, 271–289 (1982). 31. Bennett, V. Meth. Enzym. 96, 313–324 (1983). 32. Fairbanks, G., Steck, T. & Wallach, D. Biochemistry 10, 2606–2617 (1971). 33. Hunter, W. & Greenwood, F. Nature 194, 495–496 (1962). 34. Gopalakrishna, R. & Anderson, W. Biochem. biophys. Res. Commun. 104, 830–836 (1982). 35. Fabiato, A. & Fabiato, F. J. Physiol., Lond. 75, 463–505 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gardner, K., Bennett, V. Modulation of spectrin–actin assembly by erythrocyte adducin. Nature 328, 359–362 (1987). https://doi.org/10.1038/328359a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/328359a0
This article is cited by
-
Lack of adducin impairs the stability of endothelial adherens and tight junctions and may be required for cAMP-Rac1-mediated endothelial barrier stabilization
Scientific Reports (2022)
-
The conserved microRNA miR-34 regulates synaptogenesis via coordination of distinct mechanisms in presynaptic and postsynaptic cells
Nature Communications (2020)
-
Organized cannabinoid receptor distribution in neurons revealed by super-resolution fluorescence imaging
Nature Communications (2020)
-
Ultrastructure of the axonal periodic scaffold reveals a braid-like organization of actin rings
Nature Communications (2019)
-
Phosphorylation of adducin-1 by cyclin-dependent kinase 5 is important for epidermal growth factor-induced cell migration
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.