Abstract
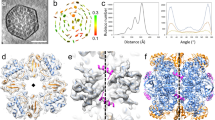
RuBPCase, D-ribulose-l,5-bisphosphate carboxylase/oxygenase (EC4.1.1.39) is the key enzyme of the reductive pentose phosphate cycle. Because of its biological significance, many structural studies on a number of plant and bacterial RuBPCases1 have been undertaken, including the enzyme isolated from the autotrophic hydrogen-oxidizing bacterium Alcaligenes eutrophus H16 (refs 2–6). Although both the higher plant enzyme and the A. eutrophus enzyme consist of eight large and eight small subunits (L8S8), no model describing the quaternary structure is generally accepted. Here we present a model for the A. eutrophus RuBPCase derived from X-ray crystallography of three-dimensional (3D) crystals, and electron microscopy and image analysis of two-dimensional (2D) crystals of the enzyme. The X-ray electron density of RuBPCase in the presence of HCO−3, Mg2+, and the transition state analogue 2-carboxyarabinitol-l,5-bisphosphate (CABP) shows an L8S8 molecule in which the L4S4 half molecules have local 4-fold symmetry (C4). The local 4-fold axes of the two L4S4 halves do not coincide but are shifted by 36 Å and are related by a crystallographic 2-fold axis perpendicular to and between the local 4-fold axes. Electron microscope data of the enzyme without CABP, which can be perfectly modelled using the X-ray densities, do not show this shift and the low-resolution point group of the molecules in the 2D crystals is D4. Both structures are presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Miziorko, H. M. & Lorimer, G. H. A. Rev. Biochem. 52, 507–535 (1983).
Bowien, B., Mayer, F., Codd, G. A. & Schlegel, H. G. Arch. Microbiol. 110, 157–166 (1976).
Purohit, K. & McFadden, B. A. Biochem. biophys. Res. Commun. 71, 1220–1227 (1976).
Bowien, B. & Mayer, F. Eur. J. Biochem. 88, 97–107 (1978).
Bowien, B. et al. Eur. J. Biochem. 106, 405–410 (1980).
Pal, G. P., Jakob, R., Hahn, U., Bowien, B. & Saenger, W. J. biol. Chem. 260, 10768–10770 (1985).
Baker, T. S., Eisenberg, D., Eiserling, F. A. & Weissman, L. J. molec. Biol. 91, 391–399 (1975).
Baker, T. S., Eisenberg, D. & Eiserling, F. A. Science 196, 293–295 (1977).
Baker, T. S., Suh, S. W. & Eisenberg, D. Proc. natn. Acad. Sci. U.S.A. 74, 1037–1041 (1977).
Bowien, B. & Gottschalk, E.-M. J. biol. Chem. 157, 11845–11847 (1982).
Meisenberger, O., Pilz, I., Bowien, B., Pal, G. P. & Saenger, W. J. biol. Chem. 259, 4463–4465 (1984).
Wang, B. C. in Lecture Notes for the School on Direct Methods and Macromolecular Crystallography, 1–7 (Medical Foundation of Buffalo, New York, 1983).
Rossmann, M. G. & Blow, D. M. Acta crystallogr. 15, 24–31 (1962).
Schlegel, H. G., Kaltwasser, H. & Gottschalk, G. Arch. Microbiol. 38, 209–222 (1961).
Friedrich, C. G. J. Bact. 149, 203–210 (1982).
Weber, K., Pringle, J. R. & Osborne, M. Meth. Enzym. 26, 3–27 (1972).
Barcena, J. A. & Shaw, P. J. Planta 163, 141–144 (1985).
Saxton, W. O. & Baumeister, W. J. Micro. 127, 127–138 (1982).
Van Heel, M. & Keegstra, W. Ultramicroscopy 7, 113–130 (1981).
Van Heel, M. & Stöffier-Meilicke, M. EMBO J. 4, 2389–2395 (1985).
Johannssen, W., Schütte, H., Mayer, F. & Mayer, H. J. molec. Biol. 134, 707–726 (1979).
Johal, S., Partridge, B. E. & Cholet, R. J. biol. Chem. 260, 9894–9904 (1985).
Van Heel, M. Ultramicroscopy 11, 307–314 (1983).
Saxton, W. O. Ultramicroscopy 16, 387–394 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holzenburg, A., Mayer, F., Harauz, G. et al. Structure of D-ribulose-l,5-bisphosphate carboxylase/oxygenase from Alcaligenes eutrophyus H16. Nature 325, 730–732 (1987). https://doi.org/10.1038/325730a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/325730a0
This article is cited by
-
Ultrastructure and cytochemistry ofPhaseolus leaf tissues infected with an isolate of tobacco necrosis virus inducing localized wilting
Protoplasma (1993)
-
Sliding-layer conformational change limited by the quaternary structure of plant RuBisCO
Nature (1987)
-
Structure of key enzyme refined
Nature (1987)
-
Do intrathylakoidal inclusions really contain RuBPCase?
Protoplasma (1987)
-
Moderne Elektronenmikroskopie auf zellul�rem und makromolekularem Niveau
Naturwissenschaften (1987)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.