Abstract
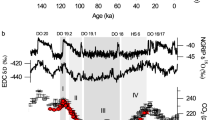
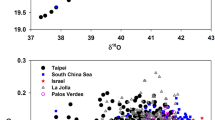
In photosynthesis, O2 is continuously formed from H2O and released to the atmosphere. Coupled with respiration, photosynthesis forms a loop in which oxygen isotopes are exchanged between O2 and H2O. During the ice ages, sea water was enriched in δ18O by ∼1.3‰ relative to the present value1. Continental waters in the areas of high primary productivity exchange rapidly with the oceans. They probably presented a similar isotopic enrichment. Since the δ18O of glacial water was greater than at present, we would expect that the δ18O of atmospheric O2 was also greater than at present. Fireman and Norris2 and Horibe et al.3 have measured the δ18O of O2 from the glacial atmosphere by analysing trapped gases in ice cores. However, their data are either too few or too imprecise to demonstrate whether δ18O of atmospheric O2 has, in fact, varied. Here we present data on the changes, during the past 22 kyr approximately, in the δ18O of atmospheric O2 trapped in the ice core Dome C (East Antarctica, 74° S, 124° E). The results show that the isotopic composition of atmospheric O2 has indeed varied along with that of sea water, and that the δ18O (O2) record offers a tool for studying several important aspects of the global cycles of O2 and H2O in relation to the climate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shackleton, N. J. Nature 215, 15–17 (1967).
Fireman, E. L. & Norris, T. L. Earth planet. Sci. Lett. 60, 339–350 (1982).
Horibe, Y., Shigehara, K. & Langway, C. Jr Earth planet. Sci. Lett. 73, 207–210 (1985).
Delmas, R. J., Ascencio, J. M. & Legrand, M. Nature 284, 155–157 (1980).
Neftel, A., Oeschger, H., Schwander, J., Stauffer, R. & Zumbrun, R. Nature 295, 220–223 (1982).
Lorius, C. & Raynaud, D. in Carbon Dioxide: Current Views and Developments in Energy/Climate Research (eds. Bach, W. et al.) 145–176 (Riedel, Dordrecht, 1983).
Lorius, C., Merlivat, L., Jouzel, J. & Pourchet, M. Nature 280, 644–648 (1979).
Raynaud, D., Delmas, R., Ascencio, J. M. & Legrand, M. Ann. Glaciol. 3, 265–268 (1982).
Labeyrie, L. D. Note CEA-N-2086, CEN Saclay, France (1979).
Kroopnick, P. thesis, Univ. California, San Diego (1970).
Craig, H. Geochim. cosmochim. Acta 12, 133–149 (1957).
Kroopnick, P. & Craig, H. Science 175, 54–55 (1972).
Duplessy, J. C., Moyes, J. & Pujol, C. Nature 286, 479–482 (1980).
Duplessy, J. C., Labeyrie, L. & Shackleton, N. J. EOS 66, 292 (1985).
Schwander, J. & Stauffer, B. Nature 311, 45–47 (1984).
Raynaud, D. & Barnola, J. M. Nature 315, 309–311 (1985).
Urey, H. C. J. chem. Soc. 562–581 (1947).
Stevens, C. L. R., Schultz, D., Van Baalen, C. & Parker, P. L. Pl. Physiol. 56, 126–129 (1975).
Lane, G. A. & Dole, M. Science 123, 574–576 (1966).
Kroopnick, P. & Craig, H. Earth planet. Sci. Lett. 32, 375–388 (1976).
Ajtay, G. L., Ketner, P. & Duvigneaud, P. in The Global Carbon Cycle (SCOPE 13) (eds Bolin, B., Degens, E. T., Kempe, S. & Keiner, P.) 129–182 (Wiley, New York, 1979).
Woodwell, G. M. et al. Science 199, 141–146 (1978).
Bramryd, T. in The Global Carbon Cycle (SCOPE 13) (eds Bolin, B., Degens, E. T., Kempe, S. & Ketner, P.) 183–218 (Wiley, New York, 1979).
Eppley, R. W. in Primary Productivity in the Sea (ed. Falkowski, P.) 230–242 (Plenum, New York, 1980).
Jenkins, W. J. Nature 300, 246–248 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bender, M., Labeyrie, L., Raynaud, D. et al. Isotopic composition of atmospheric O2 in ice linked with deglaciation and global primary productivity. Nature 318, 349–352 (1985). https://doi.org/10.1038/318349a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/318349a0
This article is cited by
-
Ice cores north and south
Nature (1994)
-
Ancient atmosphere- Validity of ice records
Environmental Science and Pollution Research (1994)
-
The role of phytoplankton photosynthesis in global biogeochemical cycles
Photosynthesis Research (1994)
-
Extending the Vostok ice-core record of palaeoclimate to the penultimate glacial period
Nature (1993)
-
N2O measurements of air extracted from antarctic ice cores: Implication on atmospheric N2O back to the last glacial-interglacial transition
Journal of Atmospheric Chemistry (1989)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.