Abstract
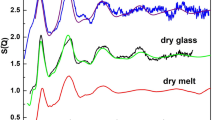
Geochemists often debate the conditions in which Al3+ may become 6-coordinated in molten silicates and how such liquid structures are related to those of coexisting crystal phases1–4. To date no experimental evidence for the occurrence of Al3+ in this coordination state has been presented and computer simulation studies have suggested that pressures exceeding 100 kbar may be required for the 4→6 conversion5,6. To examine this question in the laboratory, we have taken advantage of the ability of the glass transition to freeze the structural equilibrium of a high-pressure melt and preserve it for subsequent examination in ambient conditions. Glasses of albite composition have been prepared by quenching melts under pressures of 0–80 kbar (0–8 GPa). The Al3+ coordination has been determined by 27Al solid-state NMR spectrometry. We find that the 4-coordinated state is retained to pressures well beyond 30 kbar. A new peak at −16 p.p.m. relative to Al(H2O)3+6, which we associate with octahedral Al3+ appears weakly at 60 kbar and becomes a prominent feature of the spectrum at 80 kbar.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Waff, H. S. Geophys. J. Res. Lett. 2, 193–196 (1975).
Velde, B. & Kushiro, I. Yb. Carnegie Instn. Wash. 75, 618–620 (1975).
Sharma, S. K., Virgo, D. & Kuchiro, I. Am. Miner. 64, 779–787 (1979); J. non-cryst. Solids 33, 235–248 (1979).
De Jong, B. H. W. S., Schramm, C. M. & Paziale, V. E. Geochim. cosmochim. Acta 47, 1223–1236 (1983).
Angell, C. A., Cheeseman, P. A. & Tamaddon, S. Science 218, 885–887 (1982).
Angell, C. A., Cheeseman, P. A. & Tamaddon, S. Bull. Miner. 1–2, 87–99 (1983).
Renniger, A. L. & Uhlmann, D. R. J. non-cryst. Solids 16, 325–327 (1974).
Buccaro, J. A. & Dardy, H. D. J. non-cryst. Solids 20, 149–151 (1976).
Laberge, N. L., Vasilescu, V. V., Montrose, C. J. & Macedo, P. B. J. Am. ceram. Soc. 56, 506–509 (1973).
Landau, L. & Lifschitz, E. M., Statistical Physics. Ch. 12 (Pergamon, London, 1958).
Ohtani, E. J. Phys. Earth 27, 189–208 (1979).
Barkatt, R. & Angell, C. A. J. chem. Phys. 70, 901–911 (1979).
Müller, D. Phys. Chem. Glasses 24, 37–42 (1983).
Müller, D., Gessner, W., Behrens, H. J. & Scheler, G. Chem. Phys. Lett. 79, 59–62 (1981).
Müller, D., Hoebbel, D. & Gessner, W. Chem. Phys. Lett. 84, 25–28 (1981).
Woodcock, L. V., Angell, C. A. & Cheeseman, P. A. J. chem. Phys. 65, 1565–1577 (1976).
Kushiro, I. Geochim. cosmochim. Acta 47, 1415–1422 (1983).
Bell, P. M. & Roseboom, E. H. Miner. Soc Am. spec. Pap. 2, 151–161 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohtani, E., Taulelle, F. & Angell, C. Al3+ coordination changes in liquid aluminosilicates under pressure. Nature 314, 78–81 (1985). https://doi.org/10.1038/314078a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/314078a0
This article is cited by
-
Water-melt interaction in hydrous magmatic systems at high temperature and pressure
Progress in Earth and Planetary Science (2014)
-
Viscosity of silicate melts in CaMgSi2O6–NaAlSi2O6 system at high pressure
Physics and Chemistry of Minerals (2005)
-
Effect of high pressure on the refractive index and density of natural aluminosilicate glasses of alkali basalt composition in the SiO2-Al2O3-TiO2-Fe2O3-P2O5-FeO-MnO-CaO-MgO-Na2O-K2O system
Glass Physics and Chemistry (2004)
-
Application of27Al NMR techniques to structure determination in solids
Applied Magnetic Resonance (1993)
-
Application of infrared spectroscopy to studies of silicate glass structure: Examples from the melilite glasses and the systems Na2O-SiO2 and Na2O-Al2O3-SiO2
Journal of Earth System Science (1990)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.