Abstract
Background:
Cold application to the hand (CAH) is associated in healthy people with increase in heart rate (HR) and blood pressure (BP).
Objective:
To study hemodynamic responses to CAH in humans following spinal cord injuries of various levels, and examine the effect of spinal cord integrity on the cold pressor response.
Design:
An experimental controlled study.
Setting:
The spinal research laboratory, Loewenstein Hospital, Raanana, Israel.
Subjects:
Thirteen healthy subjects, 10 patients with traumatic T4–6 paraplegia and 11 patients with traumatic C4–7 tetraplegia.
Main outcome measures:
HR, BP, HR and BP spectral components (low frequency, LF; high frequency, HF; LF/HF), cerebral blood flow velocity (CBFV) and cerebrovascular resistance index (CVRi).
Methods:
The outcome measures of the three subject groups monitored for HR, BP and CBFV were compared from 5 min before to 5 min after 40–150 s of CAH. The recorded signals were digitized online and analyzed offline in both the time and frequency domains.
Results:
During CAH, HR and CVRi increased significantly in all subject groups (P<0.001), and BP in control subjects and in the tetraplegia group (P<0.01). BP increase was not statistically significant in paraplegia, and CBFV, HR LF, HR HF and BP LF did not change significantly during CAH in any group.
Conclusions:
The CAH effect in tetraplegia and the suppressed BP increase in paraplegia, supported by the other findings, suggest a contribution of an independent thoracic spinal mechanism to the cold pressor response.
Similar content being viewed by others
Introduction
Cold application to the hand (CAH) is followed by reflex autonomic phenomena, and has been customarily used as the ‘cold pressor test’ (CPT) to assess the autonomic nervous system (ANS). In healthy people, the afferent signals of the cold pressor reflex are thought to be mediated by cold temperature nociceptors and sensory nerve fibers, and to be integrated in the central nervous system, mainly in the hypothalamus and medullary vasomotor center. The efferent limb of the cold pressor response is thought to be mediated by the vagus and sympathetic nerves to the heart and to the peripheral blood vessels.1
This mechanism is usually reflected by heart rate (HR) acceleration and by a rise in both diastolic and systolic blood pressure (BP) in healthy people, which follow peripheral vasoconstriction and an increase in heart contractility. The normal rise is at least 10 mm Hg for diastolic BP and 15 mm Hg for systolic BP. The hemodynamic CPT effect is maximal about 60 s after limb immersion, and most people find it remarkably painful to hold their hand in freezing water for more than 120 s.1 HR and BP changes following CPT stimulation were not affected by either age or gender.2
Cerebral blood flow velocity (CBFV) showed variable response to CPT. Some studies found bilateral or unilateral (in the brain hemisphere that is contralateral (Co) to the cold applied hand) CBFV increase during CPT.3, 4, 5, 6 The increase was smaller at old age but unaffected by gender.4, 5 In one study CPT decreased CBFV.7
Spinal cord (SC) dysfunction can modulate the response to CPT. Lehmann et al.8 used the absence of cold pressor responses to confirm the completeness of autonomic lesions in patients with C5–8 tetraplegia. In 1948, Reiser and Ferris9 showed that spinal anesthesia at the T3–4 level abolished the pressor response in hypertensive patients, whereas spinal anesthesia at the T6–8 level dampened it, indicating that the SC below T4, and mainly at the T4–6 level, takes part in the CPT mechanism.
Despite this information, knowledge about CPT in patients with spinal cord injuries (SCI) is scant, and the role of the SC in the CPT response is not clear. The effect of injury level on hemodynamic responses, and the effect of SCI on HR and BP variations and on CBFV during CAH are unknown. To investigate these and examine the effect of SC integrity on the cold pressor response mechanism, we studied CAH in patients with paraplegia and tetraplegia.
Methods
Subjects
Thirteen healthy control subjects and 21 patients with SCI of 3 months to 41 years duration were included in the study. The control subjects were nine men and four women, 34±13 years old. Ten patients, eight men and two women, 38±13 years old, had T4–6 paraplegia with American Spinal Injury Association (ASIA) grade A.10 Eleven patients, all men 42±8 years old, had C4–7 tetraplegia, eight with ASIA grade A and three with ASIA grade B. The age differences between the groups were nonsignificant. None of the patients had medical conditions that might affect the results, such as febrile disease, heart failure, renal failure, diabetes mellitus or an additional neurological impairment.
Procedure
The ethics committee of Loewenstein Rehabilitation Hospital approved the study, and all participants signed an informed consent. In the morning, after a 12-h fast, each subject lay supine in a relatively quiet hospital environment with an ambient temperature of about 22°C. All subjects were continuously monitored in the supine position for HR, BP and CBFV, from 5 min before to 5 min after CAH by hand immersion in ice water for 40–150 s. The cold water was applied for 2 min in 29 subjects, for 150 s in one patient, and for 40–85 s in four subjects, who signaled discomfort.
This was one in a series of experiments performed on the same subjects, and it was preceded by 10-min 35° head up tiltings, 120 and 50 min before the CAH and by ingestion of a standard liquid meal 95 min before the CAH. Blood samples were also drawn during the experiment through an intravenous catheter that had been inserted into a cubital vein at least 150 min before the hand immersion.
Recording and analysis
For HR recording, continuous ECG traces were obtained using surface electrodes and a preamplifier A/D system (BIOPAC Systems, Santa Barbara, CA, USA). The Finapres (Ohmeda, Englewood, CO, USA) system was used for noninvasive continuous BP recording by means of a small pressure and optical cuff applied to the subjects' middle-finger. ECG and the middle-finger arterial pressure signals were simultaneously sampled online at 500 Hz. The digitized ECG signal was converted off-line into a continuous HR signal, and the digitized arterial pressure signal was low-pass filtered and resampled off-line at 10 Hz.
Processed HR and BP signals underwent time and frequency analysis. The frequency analysis (a spectral analysis of BP and HR variations) was performed after a 251-sample length median filtering. A Discrete Fourier transform was used in combination with a Welch Periodogram method to compute the power (amplitude squared) of the sampled signal fluctuations (HR and BP) as a function of their frequency.11 The integrals of the power values, between 0 and 0.17 Hz (low frequency, LF) and between 0.17 and 0.5 Hz (high frequency, HF), were calculated to obtain the low and high frequency components of the power spectrum.
A transcranial Doppler (TCD) (Smartlite, Rimed, Raanana, Israel) was used to record CBFV. The TCD probe, applied to the subjects' right temple, transmitted a 2 MHz pulsed wave through the ultrasonic window in the temporal bone. The Doppler frequency shift of the reflected wave was recorded by the device to compute the flow speed in a proximal segment of the middle cerebral artery.12 The CBFV signals were digitized online at a 2 Hz rate and submitted to off-line time-dependent analysis. The cerebrovascular resistance index (CVRi) was obtained by dividing the BP by the mean cerebral blood flow velocity (mCBFV).
Statistical analysis
The mean values of HR, BP, HR and BP LF and HF, LF/HF, mCBFV and CVRi were calculated for two time intervals: 0–5 min before ice-water hand immersion (at supine rest before CAH) and during the 40–150 s of ice-water hand immersion. Findings during cold application were compared with those during the preceding rest period. Analysis of variance with repeated measurements was used to examine the effects of cold application on the dependent hemodynamic variables within and between groups (paraplegia, tetraplegia and healthy participants), and the effects of the groups themselves on the variables. Post hoc comparisons between groups and conditions were performed to determine the specific effect of SCI on the hemodynamic variables. Correlations between changes in variables were examined using Pearson's correlation test.
Before the statistical analysis, the spectral components were subjected to a square root transformation and their ratios subjected to a natural logarithm (ln) transformation to approach normal distributions. Data were analyzed by SPSS for Windows version 11 (SPSS Inc., Chicago, IL, USA).
Results
The values of the hemodynamic variables at rest before and during CAH are shown in Tables 1, 2 and 3; the effects of CAH are summarized in Table 4.
HR
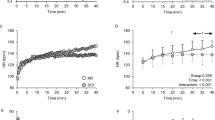
Considering all groups together, HR was significantly increased by CAH (P<0.001). HR mean values increased as a result of CAH in most patients, irrespective of specific injury level (e.g., C4, C7, T4 or T5). In the patients with tetraplegia, the increase in HR was small, but the effect of the group on the increase in HR was hardly significant (P=0.054) (Tables 1a and 4; Figure 1). Group effect on HR itself during supine rest before CAH (rest-H) and during CAH was significant (P<0.01); patients with paraplegia showed higher HR values than the other subjects.
HR power spectrum LF and HF components
The square root of the HR power spectrum LF and HF components area (HRLF and HRHF) was not significantly affected by CAH, irrespective of group (Tables 1b, c and 4; Figure 2). Group effect on the HRLF, during rest-H and CAH was significant (P<0.001): patients showed lower values than the control subjects. But group effect on HRHF at rest-H and CAH was not found significant.
BP
Overall CAH effect on BP was significant (P<0.001): BP increased in all groups after ice-water hand immersion (Tables 2a and 4; Figure 1). BP mean values increased as a result of CAH in most patients, irrespective of specific injury level (e.g., C4, C7, T4 or T5). The increase, however, was group dependent (P=0.013): it was highest in the control and the tetraplegia groups (P<0.01) and lowest in the paraplegia group, where the increase was not statistically significant. Group effect during rest-H and CAH was also significant (P=0.001); BP values were higher in the control group than in the patient groups.
BP power spectrum LF and HF component
The square root of the BP power spectrum LF component area (BPLF) was not significantly affected by CAH, irrespective of group, but was positively correlated with BP increase in the control group (r=0.684; P=0.029) and negatively correlated in the tetraplegia group (r=−0.868; P=0.002). The square root of the BP power spectrum HF component area (BPHF), however, was significantly affected by CAH (P=0.017), irrespective of group: it increased in all the groups, but mainly in patients with paraplegia and minimally in patients with tetraplegia (Tables 2b, c and 4). Group effect on BP LF, at rest-H and CAH, was significant (P<0.001): patients showed lower values than the control subjects. But group effect on BPHF at rest-H and CAH was not found to be significant.
HR and BP power spectrum LF/HF
The HR and BP LF/HF ln values were not significantly affected by ice-water hand immersion, irrespective of group (P>0.1). Group effect on HR LF/HF ln, at rest-H and CAH, was not found to be significant. But group effect on BP LF/HF ln was significant (P=0.012): patients with tetraplegia showed lower values than the control subjects.
CBFV
mCBFV values at CAH decreased in control subjects and remained almost unchanged in patients in the cerebral hemisphere ipsilateral (Ip) to the cooled hand. In the Co hemisphere it increased in all the groups. But these changes and the differences between hemispheric responses were not significant in all the subjects together and in any of the groups (Tables 3a, b and 4; Figure 1). Group effect on mCBFV during rest-H and CAH was significant (P=0.018): patients with tetraplegia showed lower values than the control subjects.
Cerebrovascular resistance
Hand ice-water immersion was followed by a significant increase in CVRi (P=0.001), irrespective of hemisphere or group (Tables 3c, d and 4). Group effect on CVRi was not significant at rest-H and at CAH.
Discussion
CAH effect
In the control group, HR and BP increased during CAH, as described in previous studies, and was related to sympathetic activation and vagal suppression by the hypothalamus and brainstem vasomotor center (BVC).1 But a significant increase was also found during CAH in HR in both patient groups, and in BP in the tetraplegia group. These findings, in patients with practically complete SCI, are not compatible with the customary cold pressor reflex model, which includes afferent sensory nerve fibers and efferent sympathetic pathways that pass through the SC,1 nor with the absence of cold pressor responses in certain previously studied patients with C5–8 tetraplegia.8
It may be argued that SCI in this study, despite being clinically complete, was sufficiently incomplete anatomically to allow the responses described above. Certain facts suggest, however, that the explanation does not lie with the incompleteness of the injuries but rather with a limitation of the customary cold response model. These facts include (1) the appearance of the described responses in most ASIA A patients in this study; and (2) the fact that in the tetraplegia patients, whose afferent and efferent reflex limbs are supposed to be damaged according to the model, BP but not HR increased during CAH significantly more than in patients with paraplegia, whose afferent reflex limb from the hand to the brainstem is intact.
Moreover, the significant BP increase in tetraplegia but not in mid-thoracic paraplegia during CAH implies that activity in the intact thoracic SC contributes to BP elevation, which is absent when the thoracic SC is damaged but can be evident despite cervical SC damage. Such activity may be initiated by afferent CAH stimulation conveyed to the thoracic SC through cervical SC segments below the complete injury (C7,8).
Afferent fibers of the CAH response, which normally pass through the SC segments C6–T1, are disconnected from the brain in patients with complete sensory lesions at the C6 segment or above, but not in patients with lower lesions, or with C5–6 lesions without a complete transection. Therefore, patients with tetraplegia in the present study, who have ASIA neurological levels of C4–7, could respond to CAH either directly, through the uppermost SC, the brainstem and the vagus, or through the isolated SC and the baroreflex mechanism, as in autonomic dysreflexia. A pressor activity at the isolated T4–6 SC is compatible with the description of pressor response abolition after spinal anesthesia at the T3–4 level, but not at the T6–8 level.9
The possibility that a neural generator located outside the brainstem activates cold pressor responses is further supported by the lack of significant change in HR LF and HF and in BP LF during CAH in any of the groups. Changes in the power spectrum of HR and BP variations, which represent sympathetic and vagal activities,13 probably depend on changes in signal oscillations in the BVC. The BVC contains an ANS oscillator discharging in a synchronized rhythmical fashion, which keeps the sympathetic nerves continuously active, maintaining vascular tone.14 The non-significant power spectrum changes during CAH may indicate that changes in the BVC oscillator activity were counteracted by the forceful baroreflex during CPT, and that an additional neural generator, which does not interfere with the LF or HF variations, contributed to the hemodynamic changes during CPT. The positive correlation of BP LF with BP increase in the control group, and its negative correlation with BP in the tetraplegia group, may support the notion that normally BVC neural generator activation makes a major contribution to BP increase, but not in the case of cervical SCI. With cervical SCI, BP may be elevated by a different mechanism (e.g., a thoracic SC neural generator that does not interfere with the BP LF or HF), whereas activation of the BVC neural generator is reduced by the baroreflex in response to the BP increase.
The significant CVRi increase, which attenuated CBFV changes following the BP increase in all subject groups, implies that CAH can increase the cerebrovascular resistance but not necessarily through SC neural transmission. The CVRi increase could be a BVC response to higher intracranial BP or a reflection of cerebrovascular autoregulation by local tissue changes.15 But CVRi increase in the patient groups in this study can alternatively suggest that during CAH, the efferent stimulus is transmitted to the cerebral vessels through upper cervical SC segments in patients with low tetraplegia or paraplegia, and through the thoracic SC and sympathetic chain in patients with upper cervical tetraplegia.
Previous studies in normal subjects, however, showed conflicting results, only some of which conform to the findings of the present study. These studies found that CPT increased or decreased CBFV, increased cerebrovascular resistance or decreased cerebral small vessels resistance.3, 4, 5, 6 Therefore, more data are needed to clarify cerebral circulation responses to CPT.
The evidence that the cold pressor response may be generated outside the brainstem by a thoracic spinal mechanism is also reinforced by findings suggesting thoracic SC involvement in responses to other stimuli, such as food ingestion16 or cold application to the foot (unpublished data).
Study limitations
As described in a previous publication,16 the design of this study imposed a few limitations. As in other human SC studies, the completeness of the SCI is based on clinical definitions (ASIA grading), which do not exclude the sparing of autonomic fibers. But the probability of interference of such sparing with findings in groups with almost-complete lesions is small.
The preceding tests, which were performed to collect important information that can be obtained only from difficult to recruit patients who match the inclusion criteria, could have confounded the findings. But the time interval between these tests and the CPT was long, and most likely it was sufficient to eliminate this effect.
Because multiple analyses increased the chance of incidental findings, only P-values <0.01 were considered significant for the main inferences.
Conclusions
The significant BP and HR increase during CAH in tetraplegia, the suppressed BP increase in paraplegia, and the lack of significant change in HR LF and HF and in BP LF are incompatible with the customary cold pressor reflex model and suggest the contribution of an independent thoracic spinal mechanism to the cold pressor response. Further research with additional patients is required to verify these findings.
References
Scharf M, Korczyn AD . Cold pressor test. In: Korczyn AD (ed). Handbook of Autonomic Nervous System Dysfunction. Marcel Decker: New York, 1995, pp 557–562.
Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI . Gender affects sympathetic and hemodynamic responses to postural stress. Am J Physiol Heart Circ Physiol 2001; 281: H2028–H2035.
Roatta S, Micieli G, Bosone D, Losano G, Bini R, Cavallini A et al. Effect of generalised sympathetic activation by cold pressor test on cerebral haemodynamics in healthy humans. J Auton Nerv Syst 1998; 71: 159–166.
Oblak JP, Zaletel M, Zvan B, Kiauta T, Pogacnik T . The effect of age on cerebrovascular reactivity to cold pressor test and head-up tilt. Acta Neurol Scand 2002; 106: 30–33.
Zvan B, Zaletel M, Pretnar J, Pogacnik T, Kiauta T . Influence of the cold pressor test on the middle cerebral artery circulation. J Auton Nerv Syst 1998; 74: 175–178.
Sohn YH . Cerebral hemodynamic changes induced by sympathetic stimulation tests. Yonsei Med J 1998; 39: 322–327.
Micieli G, Tassorelli C, Bosone D, Cavallini A, Viotti E, Nappi G . Intracerebral vascular changes induced by cold pressor test: a model of sympathetic activation. Neurol Res 1994; 16: 163–167.
Lehmann KG, Shandling AH, Yusi AU, Froelicher VF . Altered ventricular repolarization in central sympathetic dysfunction associated with spinal cord injury. Am J Cardio 1989; 63: 1498–1504.
Reiser MF, Ferris Jr EB . The nature of the cold pressor test and its significance in relation to neurogenic and humoral mechanisms in hypertension. Clin Invest 1948; 27: 156–163.
Maynard Jr FM, Bracken MB, Creasey G, Ditunno Jr JF, Donovan WH, Ducker TB et al. International standards for neurological and functional classification of spinal cord injury. American spinal injury association. Spinal Cord 1997; 35: 266–274.
Keselbrener L, Akselrod S . Selective discrete Fourier transform algorithm for time–frequency analysis: method and application on simulated and cardiovascular signals. IEEE Trans Biomedical Eng 1996; 43: 789–802.
DeWitt LD, Wechsler LR . Transcranial Doppler. Stroke 1988; 19: 915–922.
Akselrod S . Spectral analysis of fluctuations in heart rate and other cardiovascular parameters as a tool for assessment of autonomic control. In: Korczyn AD (ed). Handbook of Autonomic Nervous System Dysfunction. Marcel Decker: New York, 1995, pp 469–493.
Malpas SC . The rhythmicity of sympathetic nerve activity. Prog Neurobiol 1998; 56: 65–96.
Guyton AC, Hall JE (eds). Cerebral blood flow, the cerebrospinal fluid, and brain metabolism. In: Textbook of Medical Physiology. WB Saunders: Philadelphia, 1996, pp 783–789.
Catz A, Bluvshtein V, Pinhas I, Akselrod S, Gelernter I, Nissel T et al. Hemodynamic effects of liquid food ingestion in mid-thoracic paraplegia: is supine postprandial hypotension related to thoracic spinal cord damage? Spinal Cord 2007; 45: 96–103.
Acknowledgements
This study was supported by the Unit of Medical Services, Rehabilitation Department, Israel Ministry of Defense and by the Tel-Aviv University Research Fund. The Transcranial Doppler device was provided for the study by Rimed Ltd, Israel. We thank Mrs Ora Philo and the nursing team of the Spinal Rehabilitation Department in Loewenstein Hospital for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catz, A., Bluvshtein, V., Pinhas, I. et al. Cold pressor test in tetraplegia and paraplegia suggests an independent role of the thoracic spinal cord in the hemodynamic responses to cold. Spinal Cord 46, 33–38 (2008). https://doi.org/10.1038/sj.sc.3102055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102055
Keywords
This article is cited by
-
A comparison of static and dynamic cerebral autoregulation during mild whole-body cold stress in individuals with and without cervical spinal cord injury: a pilot study
Spinal Cord (2018)
-
Cold pressor test in spinal cord injury—revisited
Spinal Cord (2018)
-
Cerebrovascular autoregulation: lessons learned from spaceflight research
European Journal of Applied Physiology (2013)
-
Severity of autonomic dysfunction in patients with complete spinal cord injury
Clinical Autonomic Research (2012)
-
Insulin resistance in tetraplegia but not in mid-thoracic paraplegia: is the mid-thoracic spinal cord involved in glucose regulation?
Spinal Cord (2011)