Abstract
Study design:
Prospective assessment of cardiovascular parameters in individuals with spinal cord injury (SCI) in response to harness application and postural changes including orthostatic stress.
Objective:
To evaluate arterial blood pressure and heart rate (HR) with and without harness application during sitting, supine, and standing positions in able-bodied and SCI individuals.
Methods:
Measurements were obtained in all SCI research participants (n=11) before a locomotor training intervention and compared to data with able-bodied individuals (n=9). During standing, all research participants wore a harness and were suspended by an overhead, pneumatic body weight support system.
Results:
Resting arterial blood pressure and HR in individuals with cervical SCI were significantly lower during sitting than in thoracic SCI and able-bodied individuals (P<0.05). Orthostatic stress significantly decreased arterial blood pressure only in individuals with cervical SCI (P<0.05). Harness application had no effect on cardiovascular parameters in able-bodied individuals, whereas diastolic blood pressure was significantly increased in those with SCI. Orthostatic changes in cervical SCI when sitting were ameliorated by harness application. However, while standing with harness, individuals with cervical SCI still developed orthostatic hypotension.
Conclusions:
Level of injury to the spinal cord influences baseline cardiovascular parameters. Application of harness in individuals with SCI could alter baseline cardiovascular parameters and the response to orthostatic stress. This should be carefully considered when assessing effects of therapeutic interventions using body weight support in individuals with SCI.
Sponsorship:
Christopher Reeve Foundation HA2-0201-2B; Roman Reed Spinal Cord Injury Research Fund of California RR 04084532.
Similar content being viewed by others
Introduction
Abnormal cardiovascular control following spinal cord injury (SCI) is a well-documented phenomenon in humans and in animal models.1, 2, 3, 4, 5, 6, 7 Persistent low resting arterial blood pressure is prevalent after cervical SCI and, to a lesser extent, after upper thoracic SCI.8 However, individuals with both types of injury will often experience further reductions in arterial blood pressure in the upright posture (orthostatic hypotension).9, 10, 11, 12 Futhermore, clinical observations suggest that SCI has been associated with an increased risk of mortality from cardivascular diseases including both ischemic and non-ischemic heart disease.13 Decreased mobility, lack of exercise and unstable cardiovascular control in individuals with SCI are major contributors to the risk of development of cardiovascular diseases in this population. Unfortunately, when exercise programs are implemented in these individuals, the unstable cardiovascular control could also be a factor that prevents them from participation. Further, the presence of orthostatic hypotension significantly hampers and prolongs the rehabilitation period, increases length of hospitalization, and in many cases results in significant financial burden for the individuals and society.14
Orthostatic hypotension is a common problem in individuals with quadriplegia and paraplegia, particularly in the acute phase.10 Orthostatic hypotension does improve over time, although the reasons for this improvement have not been clearly established; potential mechanisms include vascular wall receptor hypersensitivity, increased skeletal muscle tone, some recovery of postural reflexes at a spinal level, adaptation of the renin–angiotensin system, or other unknown mechanisms.15
Locomotor Training (LT) using body weight support and a treadmill that practices standing and stepping has evolved from animal and human studies that indicate spinal interneuronal networks are highly dependent on continuous afferent feedback specific to these motor tasks.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Standing is considered beneficial for people with SCI who are confined to a wheelchair, as immobilization can contribute to secondary pathologies such as osteoporosis, leg muscle contractures, pressure sores, and muscle atrophy.29, 30
However, little is known of the effect of body weight support training or standing on cardiovascular control in individuals with motor complete SCI. The ultimate goal is to evaluate whether stand LT training results in neuromuscular activation as well as activation of spinal autonomic circuits resulting in amelioration of abnormal cardiovascular control and specifically orthostatic hypotension in individuals with complete SCI. In this study, we evaluated the effects of harness application and changes in posture on cardiovascular parameters of SCI and able-bodied individuals.
Methods
Research participants
The UCLA Institutional Review Board approved the experimental protocol for the present study and each subject signed an informed consent form before participating in the study. Nine able-bodied individuals and 12 individuals with SCI volunteered for this study (Table 1). A clinician assessed the level and extent of SCI according to the American Spinal Injury Association (ASIA/IMSoP) impairment scale.31 All research participants were classified as having a clinically complete SCI, as they were graded as ASIA A (no motor or sensory function below the lesion including the sacral segments S4–S5). These individuals had a stable medical condition without underlying cardiopulmonary disorders. Able-bodied individuals were healthy with no history of cardiopulmonary disease. None of the research participants were taking any antispasticity or vasoactive medications during the study and were free from caffeine on the day of evaluation.
Experimental design
In all research participants (n=20), we measured arterial blood pressure and heart rate (HR) during different postural positions both with and without the application of a harness (Figure 1). Hemodynamic measurements were obtained during each event at 1-min intervals for 5 min for each event. The sequence of events were as follows: (1) sitting without a harness in their wheelchair (S); (2) lying supine on a mat table without a harness (SUP); (3) lying supine on a mat table immediately after harness application (SUP-H); (4) sitting with the harness applied in their wheelchair (S-H); and (5) standing with the harness applied with body weight support (ST-H). This sequence of postural changes (sitting to supine, supine to sitting, sitting to standing) was necessary to eliminate multiple stages of transfers of patients and for the practical application of the harness before using the body weight support system (Figure 2). Individuals did not exercise, walk, or propel their wheelchair for at least 10 min before the baseline hemodynamic measurements. During standing, able-bodied (n=9) and SCI research participants (n=11) wore a harness and were suspended by an overhead, pneumatic BWS system (Vigor, Stevensville, MI, USA). SCI research participants were provided with manual assistance by trainers as necessary at the trunk and legs.
Harness application. Harness was placed on individuals in a supine position with straps located at the lower chest and upper abdomen (a). The pelvic band and legs straps were fastened to avoid slippage (b). The specialized harness was designed by Robertson® (Las Vegas, NV, USA) and was used in previous studies of locomotor training interventions.16, 17 The modified climbing harness was designed to distribute the forces throughout the chest and pelvic bands and reduce the force through the straps. Forces specific to regions of the harness were not measured; however, participants most often commented on pressures at the edges of the chest band and pelvic straps. The harness was immediately adjusted to relieve excess pressure
The sequence of events during hemodynamic data collection for able-bodied and SCI research participants. Arterial blood pressure and HR were measured during sitting without a harness (S), lying supine without a harness (SUP), lying supine with the harness (SUP-H), sitting with the harness (S-H), and standing with the harness (ST-H). Harness application is indicated by an arrow
Data acquisition and analyses
Blood pressure was measured manually by the same examiner using a standard calibrated sphygmomanometer (Tycos 509 Mobile Aneroid, Welch Allyn Inc.). HR was obtained using an oxymeter (Nonin Onyx 9500, Nonin Medical Inc.) on the index finger of the opposite arm from the blood pressure measurements. Data were used from the 5 min measurement for all hemodynamic parameters. MAP was calculated using the formula (DBP+(SBP−DBP)/3). In the present study, orthostatic hypotension was identified by using criteria established by the American Autonomic Society and the American Academy of Neurology: patients who had systolic blood pressure drop of 20 mmHg or greater or diastolic blood pressure drop of 10 mmHg or greater within 3 min of sitting up were designated as orthostatically hypotensive.32
Statistical analysis
Data were statistically analyzed using SigmaStat for Windows Version 2.03 (SSPS Inc., Chicago, IL, USA). Paired t-tests were used to identify significant differences in hemodynamic parameters between the experimental events. Differences among cervical SCI, thoracic SCI, and able-bodied individuals were assessed using one-way ANOVA. Post hoc analysis of means was performed using the Tukey–Kramer test. An alpha level of 0.05 was used for statistical significance.
Results
Demographic data of participants
Eleven individuals with a cervical (n=6) or thoracic (n=5) SCI and nine able-bodied individuals participated in the study. The majority of SCI individuals were male (92%) with age range 21–55 years (average 31.8±11.3 years; Table 1). All individuals sustained a traumatic SCI in our study. The average time post-injury was 5.8±8.3 years. Able-bodied individuals (four male, five female) were in the age range 19–48 years (average 23.4±9.3 years). There was no difference in average age between able-bodied and SCI individuals.
Effect of harness application on cardiovascular parameters
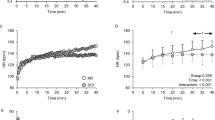
Average systolic and diastolic arterial blood pressures were lower in individuals with cervical SCI (86±7/54±7 mmHg) than thoracic SCI (106±8/65±6 mmHg) and able-bodied individuals (109±6/69±3 mmHg; P⩽0.05). Able-bodied individuals had minimal changes in hemodynamic parameters following harness application in the supine position or when sitting with and without the harness were compared (Figure 3). In individuals with thoracic SCI, there was no immediate effect of harness on cardiovascular parameters when applied in the supine position; however, diastolic pressure was significantly higher during sitting with the harness. In individuals with cervical SCI, only diastolic pressure increased with application of harness while in supine position; however, while sitting in the wheelchair, the harness application significantly increased arterial blood pressure.
Cardiovascular parameters in control and individuals with cervical (C-SCI) and thoracic (T-SCI) injury during different stages of study: sitting position at rest (S), supine (SUP), supine with harness (SUP-H), sitting with harness (S-H), and standing with harness (ST-H). Significant differences (P<0.05) between with and without harness application (left panel) and between designated postural positions (right panel) are denoted with an *. Clinically relevant differences are denoted with #
Effect of postural changes on baseline cardiovascular parameters
Able-bodied individuals had significant decreases in diastolic blood pressure and HR when moving from sitting to supine position (Figure 3). Individuals with thoracic SCI had no response in cardiovascular parameters when moved to the supine position. In contrast, individuals with cervical SCI had a significant (P<0.05) increase in systolic blood pressure and significant (P<0.05) decrease in HR in supine compared to sitting positions. Able-bodied and thoracic SCI individuals responded to orthostatic stress induced by the body weight support system raising them to the upright standing position with increases in diastolic pressure and HR. Individuals with cervical SCI suffered orthostatic hypotension with statistically and clinically significant decreases in arterial blood pressure. Noticeable bradycardia was present in all individuals with cervical SCI in sitting (75±10 b.p.m.) and standing positions (80±6 b.p.m.).
Discussion
Baseline cardiovascular parameters
In our present study, basal arterial blood pressure in sitting position was significantly lower in individuals with cervical SCI than in thoracic and able-bodied controls. Low arterial blood pressure is a common problem in both acute and chronic high-level SCI patients. It was reported previously that there is an inverse linear relationship between the level of SCI and resting blood pressure.6, 8, 11 The reduction in sympathetic drive below the level of SCI is thought to be responsible for the lower resting blood pressure in individuals with SCI.5 Frankel and Mathias33 recorded the resting blood pressure of 461 male patients with traumatic complete SCI and observed an inverse linear relationship between the level of SCI and resting blood pressure. In another study, Lehmann et al34 presented similar data in 71 consecutive patients admitted to a neurological intensive care unit within 12 months of SCI. The resting supine blood pressure of patients with complete cervical SCI was lower than that of normal individuals; the resting supine blood pressure of patients with lower thoracic and lumbar lesions was similar to control individuals.34
Cardiovascular responses to postural changes
When able-bodied individuals assume upright posture, this activates a cascade of autonomic and hemodynamic adjustments: right and left stroke volumes decline, HR increases owing to vagal withdrawal; in contrast, there is an increase in sympathetic activation resulting in increased peripheral resistance.35, 36 These responses prevent the displacement of blood volume towards the lower body and allow to maintain a sufficient brain perfusion on assumption of upright position. In able-bodied individual, the normal response to standing is usually limited to minimal hemodynamic changes: a fall in systolic blood pressure ranging from 5 to 10 mmHg, a similar increase in diastolic blood pressure, as well as an increase in HR of 10–25 b.p.m.37
However, in individuals with SCI, in addition to the low basal arterial blood pressure that is a feature of SCI, they also experience further drops in blood pressure on assuming the upright posture (known as orthostatic hypotension).9, 11, 15
Presently, orthostatic hypotension is considered when systolic blood pressure decreases by at least 20 mmHg and diastolic by at least of 10 mmHg within 3 min of standing or the trunk being elevated to a position of more than 60°.32 Individuals usually report lightheadedness, dizziness, and blurred vision with episodes of orthostatic hypotension.38 Illman et al14 reported that 58.9% of SCI individuals who developed orthostatic hypotension presented with these symptoms. Frisbie and Steele39 reported dizziness and fainting in 100% of patients with orthostatic hypotension owing to SCI and lesion rostral to T7. It is important to mention that other symptoms of orthostatic hypotension, including fatigue or weakness (67%), could be very disabling for these individuals, preventing them from rehabilitation or even activities of daily living.39
Effect of abdominal compression on cardiovascular parameters
As we indicated in the introduction, the main goal of this study was to examine the effect of harness application of baseline cardiovascular parameters in individuals with SCI before making any conclusions of effects of body weight support training on cardiovascular parameters. Harness applications for the support of SCI individual for standing in some ways are similar to the abdominal binders that were previously utilized for management of orthostatic hypotension in different conditions including SCI.6, 40, 41 The splanchnic circulation could contain up to 25% of blood volume at rest and consists of large highly complaint venous bed.42 In individuals with autonomic dysfunctions, including individuals with SCI, impaired splanchnic autonomic control leads to increased splanchnic blood volume with orthostatic challenge.6
A variety of pharmacological and non-pharmacological methods are presently used in order to decrease orthostatic hypotension and to ameliorate related symptoms.15, 30, 41, 43 One of the non-pharmacological alternatives commonly used in individuals with orthostatic hypotension is the application of external counterpressure in order to decrease capacitance of the vasculature beds in legs and abdominal cavity – the major areas of blood pool during standing.40, 41, 44, 45, 46 Previously, numerous studies evaluated effects of chest and abdominal binders on cardiovascular parameters owing to increased intrathoracic pressure.47, 48 The authors reported that changes in intrathoracic pressure were associated with reciprocal changes in both intrathoracic vascular pressures and blood volume. For example, in animals with acute ventricular failure, stroke volume increased with increasing introthoracic pressure.47
We also would like to acknowledge the possible effects of abdominal binders on respiratory modulation and blood pressure. In individuals with low cervical SCI, spontaneous respiration is dependent mostly on diaphragmatic breathing.49, 50 When individuals with SCI are in a supine position, the diaphragm has increased inspiratory excursion owing to more favorable muscle tension with abdominal content pressing on the diaphragm.49 Therefore, the presence of the abdominal binder supports the paralyzed abdominal muscles and relocates abdominal content close to the diaphragm resulting in increased contractile capacity of the diaphragm. Furthermore, additional abdominal pressure redistributes blood from abdominal content to the circulatory system. However, it was shown in able-bodied individuals that diaphragmatic breathing by itself results in increased intra-abdominal pressure. Diaphragmatic breathing in able-bodied individuals also impedes venous flow in femoral veins and abolishes venous return over the last three-quarters of a breath.51 Therefore, an abdominal binder could further increase intra-abdominal pressure and compromise the venous return to the thorax.
Furthermore, studies with use of an anti-gravity suit that increased lower-body positive pressure in individuals with SCI also demonstrated an increase of cardiac output and stroke volume.40, 52 For symptomatic treatment, numerous investigators reported benefit of abdominal binders in individuals with orthostatic hypotension owing to various disorders including SCI.52, 53 Also, the application of abdominal binders has reduced dizziness, lessened blurred vision, and decreased fatigue in the sitting or the standing position in these individuals.
Denq et al45 reported that compression of the abdomen is by far the most effective site to improve standing arterial pressure in patients with orthostatic hypotension. More recently, Smit et al41 in a cohort of 23 individuals with orthostatic hypotension of varying etiology also demonstrated that abdominal compression significantly improves upright blood pressure by an increase in stroke volume. In individuals with SCI, the data are somewhat inconsistent. Some investigators reported that abdominal binders did not alter physiological measures,44 whereas others indicated that abdominal binders or stockings improve cardiovascular as well as respiratory parameters.40, 54 Our data demonstrated that harness application did not change cardiovascular parameters in able-bodied controls and individuals with thoracic SCI. There was a significant increase in arterial blood pressure in individuals with cervical SCI in sitting position following harness application. These findings are not surprising and are similar to previous observations with improved homodynamic parameters with abdominal binders in SCI individuals.40, 52 Interestingly, despite the use of the harness, individuals with cervical SCI were not able to maintain their arterial blood pressure following standing. This signifies that harness application did not prevent development of orthostatic hypotension in individuals with cervical SCI. The failure of the harness to support arterial blood pressure following standing and resulted orthostatic hypotension appears to be related to numerous factors including excessive pooling of blood in the viscera and lower extremities, presumably owing to the absence or low level of tonic sympathetic activity below the lesion.11 This is likely to be compounded by the loss of function and atrophy of lower extremity muscle that are known to be important in counteracting venous pooling in the upright position despite the low venous compliance reported in individuals with SCI.12, 55 The altered cardiovascular responses during orthostatic stress in subjects with SCI do not seem to be the product of altered venous compliance but are a result of a combination of numerous factors: possible skeletal muscle and venous vascular morphological changes, alteration in baroreceptor sensitivity, and failure of redistribution of the blood volume.
Limitations of the study
The authors would also like to outline some limitations of the study. In addition to disrupted sympathetic outflow and deconditioning, other factors could contribute to the orthostatic instability in individuals with SCI. For example, medication, presence of the peripheral neuropathy, decreased circulatory volume, and low sodium concentration are among common causes of orthostatic hypotension.15 Although we did not evaluate circulatory volumes, or performed nerve conduction studies on our participants, inclusion criteria were restricted to individuals with no history of diabetes or other neurological disorders and was on those with stable medical condition within the last 6 months. Individuals with use of vasoactive medications for management of blood pressure were also excluded from this study.
We would also like to mention that the protocol for the orthostatic testing was somewhat unusual (Figure 2). The order of the positions was chosen for the convenience of participants and limited unnecessary transfers for participants. Unfortunately, we did not have the opportunity for continuous measurements of the arterial blood pressure and HR in our study. Therefore, we could predict that in some instances, the peak of decrease or increase in blood pressure or HR was missed with the sampling at 1 min intervals. However, we believe that this small artifact will not undermine our findings with respect to observed effects of harness application on cardiovascular parameters in our subjects.
Conclusions
Numerous factors could contribute to unstable blood pressure control with significant swings from low basal blood pressure to episodes of hypertension, in individuals with SCI. Low sympathetic tone, decrease of mobility, and deconditioning are among these factors. Before evaluating the effects of body weight support training on cardiovascular parameters, the authors examined an important technical aspect of this intervention: harness aplication. Application of harness in individuals with SCI could alter baseline cardiovascular parameters and response to orthostatic stress before training. These findings could be particularly valuable and have to be carefully considered while conducting clinical trials and assessing effects of therapeutic interventions in individuals with SCI.
References
Krassioukov AV, Johns DG, Schramm LP . Sensitivity of sympathetically correlated spinal interneurons, renal sympathetic nerve activity, and arterial pressure to somatic and visceral stimuli after chronic spinal injury. J Neurotrauma 2002; 19: 1521–1529.
Krassioukov AV, Furlan JC, Fehlings MG . Autonomic dysreflexia in acute spinal cord injury: an under-recognized clinical entity. J Neurotrauma 2003; 20: 707–716.
Krenz NR, Meakin SO, Krassioukov AV, Weaver LC . Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 1999; 19: 7405–7414.
Mayorov DN, Adams MA, Krassioukov AV . Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma 2001; 18: 727–736.
Teasell RW, Arnold JM, Krassioukov A, Delaney GA . Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000; 81: 506–516.
Mathias CJ, Frankel HL . Autonomic disturbances in spinal cord lesions. In: Bannister R, Mathias CJ (eds). Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. 4th edn. Oxford University Press: Oxford 1999, pp 839–881.
Osborn JW, Taylor RF, Schramm LP . Chronic cervical spinal cord injury and autonomic hyperreflexia in rats. Am J Physiol 1990; 258: R169–R174.
Sheel AW, Krassioukov AV, Inglis JT, Elliott SL . Autonomic dysreflexia during sperm retrieval in spinal cord injury: influence of lesion level and sildenafil citrate. J Appl Physiol 2005; 99: 53–58.
Cariga P, Ahmed S, Mathias CJ, Gardner BP . The prevalence and association of neck (coat-hanger) pain and orthostatic (postural) hypotension in human spinal cord injury. Spinal Cord 2002; 40: 77–82.
Corbett JL, Debarge O, Frankel HL, Mathias C . Cardiovascular responses in tetraplegic man to muscle spasm, bladder percussion and head-up tilt. Clin Exp Pharmacol Physiol 1975; 3 (Suppl 2): 189–193.
Mathias CJ . Orthostatic hypotension: causes, mechanisms, and influencing factors. Neurol 1995; 45: S6–S11.
Wecht JM, de Meersman RE, Weir JP, Spungen AM, Bauman WA . Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clin Auton Res 2003; 13: 433–438.
DeVivo MJ, Krause JS, Lammertse DP . Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999; 80: 1411–1419.
Illman A, Stiller K, Williams M . The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord 2000; 38: 741–747.
Claydon VE, Steeves JD, Krassioukov A . Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2006; 44: 341–351.
Behrman AL, Harkema SJ . Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 2000; 80: 688–700.
Beres-Jones JA, Harkema SJ . The human spinal cord interprets velocity-dependent afferent input during stepping. Brain 2004; 127: 2232–2246.
Dietz V, Harkema SJ . Locomotor activity in spinal cord-injured persons. J Appl Physiol 2004; 96: 1954–1960.
Edgerton VR et al. Topical review: retraining the injured spinal cord. J Physiol 2001; 533: 15–22.
Harkema SJ . Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist 2001; 7: 455–468.
Harkema SJ et al. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 1997; 77: 797–811.
Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ . Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma 2002; 19: 1217–1229.
Dobkin BH et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair 2003; 17: 153–167.
Barbeau H . Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair 2003; 17: 3–11.
Barbeau H, Visintin M . Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch Phys Med Rehabil 2003; 84: 1458–1465.
Barbeau H, Fung J . The role of rehabilitation in the recovery of walking in the neurological population. Curr Opin Neurol 2001; 14: 735–740.
Barbeau H, Norman K, Fung J, Visintin M, Ladouceur M . Does neurorehabilitation play a role in the recovery of walking in neurological populations? Ann NY Acad Sci 1998; 860: 377–392.
Rossignol S et al. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp 1996; 56: 449–463.
Saitoh E et al. Clinical experience with a new hip-knee-ankle-foot orthotic system using a medial single hip joint for paraplegic standing and walking. Am J Phys Med Rehabil 1996; 75: 198–203.
Faghri PD, Yount JP, Pesce WJ, Seetharama S, Votto JJ . Circulatory hypokinesis and functional electric stimulation during standing in persons with spinal cord injury. Arch Phys Med Rehabil 2001; 82: 1587–1595.
Maynard FM et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997; 35: 266–274.
The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, multiple system atrophy. Neurology 1996; 46: 1470.
Frankel HL, Mathias CJ . The cardiovascular system in tetraplegia and paraplegia. In: Vinkin PJ, Bruyn GW, Klawans HL, Frankel HL (eds). Spinal Cord Trauma. Elsevier: Amsterdam, New York 1992, pp 313–333.
Lehmann KG, Lane JG, Piepmeier JM, Batsford WP . Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J Am Coll Cardiol 1987; 10: 46–52.
Wallin BG, Burke D, Gandevia S . Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 1994; 474: 331–338.
Cooke WH et al. Human responses to upright tilt: a window on central autonomic integration. J Physiol 1999; 517 (Part 2): 617–628.
Smit AA, Halliwill JR, Low PA, Wieling W . Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol 1999; 519 (Part 1): 1–10.
Sclater A, Alagiakrishnan K . Orthostatic hypotension. A primary care primer for assessment and treatment. Geriatrics 2004; 59: 22–27.
Frisbie JH, Steele DJ . Postural hypotension and abnormalities of salt and water metabolism in myelopathy patients. Spinal Cord 1997; 35: 303–307.
Hopman MT, Monroe M, Dueck C, Phillips WT, Skinner JS . Blood redistribution and circulatory responses to submaximal arm exercise in persons with spinal cord injury. Scand J Rehabil Med 1998; 30: 167–174.
Smit AA et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004; 14: 167–175.
Rowell LB . Human Cardiovascular Control. Reflex Control During Orthostasis. Oxford University Press: New York 1993, pp 37–80.
Jacobsen TN, Nielsen HV, Kassis E, Amtorp O . Subcutaneous and skeletal muscle vascular responses in human limbs to lower body negative pressure. Acta Physiol Scand 1992; 144: 247–252.
Kerk JK et al. Effect of an abdominal binder during wheelchair exercise. Med Sci Sports Exerc 1995; 27: 913–919.
Denq JC et al. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res 1997; 7: 321–326.
Tanaka H, Thulesius O, Borres M, Yamaguchi H, Mino M . Blood pressure responses in Japanese and Swedish children in the supine and standing position. Eur Heart J 1994; 15: 1011–1019.
Pinsky MR, Matuschak GM, Klain M . Determinants of cardiac augmentation by elevations in intrathoracic pressure. J Appl Physiol 1985; 58: 1189–1198.
Niemann JT, Rosborough JP, Criley JM . Continuous external counterpressure during closed-chest resuscitation: a critical appraisal of the military antishock trouser garment and abdominal binder. Circulation 1986; 74: IV102–IV107.
Baydur A, Adkins RH, Milic-Emili J . Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol 2001; 90: 405–411.
Bluechardt MH, Wiens M, Thomas SG, Plyley MJ . Repeated measurements of pulmonary function following spinal cord injury. Paraplegia 1992; 30: 768–774.
Miller JD, Pegelow DF, Jacques AJ, Dempsey JA . Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 2005; 563 (Part 3): 925–943.
Bhambhani Y . Physiology of wheelchair racing in athletes with spinal cord injury. Sports Med 2002; 32: 23–51.
Tanaka H, Yamaguchi H, Tamai H . Treatment of orthostatic intolerance with inflatable abdominal band. Lancet 1997; 349: 175.
Goldman JM, Rose LS, Williams SJ, Silver JR, Denison DM . Effect of abdominal binders on breathing in tetraplegic patients. Thorax 1986; 41: 940–945.
Desmond JW . Blood volume and capacitance vessel compliance in the quadraplegic patient. Can Anaesth Soc J 1974; 21: 421–426.
Acknowledgements
This work was supported by Christopher Reeve Paralysis Foundation HA2-0201-2B, Roman Reed Spinal Cord Injury Research Fund of California RR 04084532, and NIH 49209 and 16333 (PI Dr S Harkema). We acknowledge the support of collaboration between our laboratories by the grant initiative of Christopher Reeve Paralysis Foundation (PI Dr A Krassioukov). We gratefully acknowledge Claudia Angeli, Christie Ferreira, Patrick Hu, and Rubia van den Brand for support in manuscript preparation. We thank the research volunteers for their dedication and valuable contribution to this study.
Author information
Authors and Affiliations
Additional information
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research
Rights and permissions
About this article
Cite this article
Krassioukov, A., Harkema, S. Effect of harness application and postural changes on cardiovascular parameters of individuals with spinal cord injury. Spinal Cord 44, 780–786 (2006). https://doi.org/10.1038/sj.sc.3101952
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101952
Keywords
This article is cited by
-
Autonomic dysreflexia associated with cervical spinal cord gliofibroma: case report
BMC Neurology (2021)
-
Non-pharmacological interventions in patients with spinal cord compression: a systematic review
Journal of Neuro-Oncology (2018)
-
Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis
Spinal Cord (2012)
-
International spinal cord injury cardiovascular function basic data set
Spinal Cord (2010)
-
Hypoventilation during passive leg movement in spinal cord-injured humans
Clinical Autonomic Research (2010)