Abstract
Study design:
Case report.
Objective:
To present a rare pathology causing a common disease.
Setting:
Spine unit of the orthopaedic surgery department of a university hospital in Berlin/Germany.
Case report:
A 39-year-old female with an intraspinal extradural arachnoid cyst of the lumbar spine presented with intermittent radiating lumbar pain. The magnetic resonance imaging (MRI) showed a dorsal spinal extradural arachnoid cyst at L3/4. After wide laminotomy L3, operative cyst resection and stabilisation at L3/4 by posterior lumbar interbody fusion (PLIF), major symptom relief occurred.
Conclusion:
Spinal extradural arachnoid cysts are a rare entity causing low back pain and intermittent radicular syndromes. They can be caused by arachnoid herniation through dural weak spots which are hereditary or occur after trauma. A ball-valve mechanism promotes growth. The main diagnostic tool for spinal extradural cysts is the MRI scan. Additionally, myelography is helpful to demonstrate fluid communication. Complete surgical removal of the cyst should be attempted to reduce risk of recurrence. If extensive decompression is needed for the surgical approach causing segmental instability, interbody fusion is recommended. The outcome depends on age, duration and degree of neurological damage.
Similar content being viewed by others
Introduction
Spinal meningeal (arachnoid) cyst is a differential diagnosis as a cause of severe back pain with neurological symptoms. Since the incidence is rare, diagnosis of spinal meningeal cysts often occurs after several months of therapy-resistant back pain.
The pathogenesis of spinal meningeal cysts remains unsolved. They can be congenital and then derive from neuroendothelial tissue. Furthermore, post-traumatic1 and postinflammatory cases have been presented.2 They are intradural or extradural. Spinal extradural cysts mostly occur in the mid to low thoracic spine (65%) followed by the lumbar spine (12%), the sacral spine (6.6%), and the cervical spine (3.3%).3 They become symptomatic by increasing pressure on neuronal structures.
Only a small number of cases with spinal meningeal cysts causing spinal stenosis and neurological symptoms have been reported so far and therapeutic approaches have been as diverse as simple percutaneous aspiration to decompression by laminectomy and cystectomy.
We present a case of radiculopathy caused by a spinal meningeal cyst.
Case report
A 38-year-old female presented a with 9-year history of lumbar back pain radiating into the left leg. She had hyposensitivity on the left lateral thigh but no other neurological signs. Since conservative treatment was without success, she was treated psychosomatically for dysthymia. Magnetic resonance imaging (MRI) showed a cystic dural formation at L3/4. The existence of the cyst was not seen as a cause of the presented symptoms. The patient had been hospitalised on several occasions because of back pain, but surgery was never an option. After 6 months, dorsal decompression with fenestrotomy at L3/4 and L4/5 and cyst aspiration resulted in pain alleviation for a short time. However, a few days later, the pain recurred, and the patient was then treated conservatively with pain medication and manual iliosacral joint mobilisation. After 8 months, the patient presented in our outpatient clinic.
She was now a pain-burdened patient who needed crutches for walking distances longer than 20 m. She had a painful paravertebral muscular dysbalance and reduced lumbar mobility. Reflex patterns and muscle strength were normal. She had a sensory deficit L4 on the left side and iliosacral joint pain.
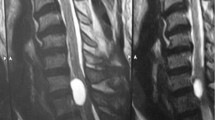
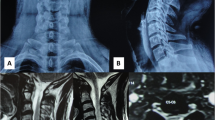
The MRI showed a meningeal cyst at L3/4 Type IA4 (Figure 1). A computed tomography (CT)-directed myelograpy of the cyst showed communication between the cyst and the free liquor space. If there had been no communication present, the cyst could have been desolated, that is, by alcohol instillation. At day 1 after cystic fluid aspiration, followed by pain reduction, the severe back pain recurred. Radiological lumbar spine imaging in ante- and retroflexion showed segmental instability at L3/4. Thus, the interspinous ligament at L3/4 was resected for a clear access and wide decompression by laminotomy was performed. The cyst was mobilised, opened and resected completely. The dorsal communicating channel was closed by direct dural closure (Figure 2). Furthermore, a posterior lumbar interbody fusion (PLIF) at L3/4 using titanium cages, cancellous bone from the iliac crest and a dorsal instrumentation (Moss Max, DePuy, UK) was performed to stabilise this segment (Figure 3). Postoperatively, the patient experienced pain reduction and relief of radicular pain, but lumbar pain chronification required continuation of pain medication (morphine, carbamazepine). At the same time, she was treated with intensive physiotherapy including stabilising spinal muscle training. Over the 2-year-follow–up, the patient still had muscular dysbalance causing back pain. The symptoms decreased steadily and pain medication is not needed anymore.
Discussion and conclusion
Intraspinal meningeal cysts are a heterogenous entity which can be classified by CT-myelography or MRI combined with histological and intraoperative findings using the Nabors classification.4 Type I lesions are extradural meningeal cysts without spinal nerve root fibres, which can be subdivided into Type IA extradural meningeal cysts and Type IB sacral meningocele. Type II meningeal cysts are extradural and contain nerve root fibres (‘Tarlov's perineural cyst’). Type III includes all intradural arachnoid cysts.
The etiology and pathogenesis of extradural arachnoid cysts have been discussed controversially in the available literature. Rohrer et al5 proposed a herniation of the arachnoid layer through a hereditarily or inflammatorily/traumatically induced dural weak spot. A ball-valve mechanism would promote growth of the cyst by causing symptomatic compression of neural structures. Others suggested a congenital diverticulum of the dura mater, which grows by active and passive fluid transport.6 Congenital lesions have been found in children in association with neural tube defects.7 Fortuna et al8 suggested an incarceration of arachnoid granulations growing through cerebrospinal fluid production, which becomes entrapped in the arachnoid diverticula. In the presented case, the origin of the cyst remains unclear.
Spinal cysts mostly become evident through nerve compression symptoms. They can be intermittent or slowly progressive. By increasing intraspinal pressure, the symptoms get worse. Type and amount of neurological deficits depend on the location and size of the cyst. Thoracic cysts often result in girdle-like radicular thoracic pain while lumbar cysts, as seen in the presented case, mostly cause low back pain associated with radicular radiation with and without sensomotor deficits. Sacral cysts sometimes cause bowel and bladder dysfunction.9 Since the symptoms rarely are persistent, intermittent diagnosis and treatment is often delayed. Spinal abnormalities are associated with a higher incidence of spinal meningeal cysts.4 The clinical findings should lead to diagnostic imaging. Plain X-rays can reveal hereditary bone defects. Myelography can be helpful in detecting spinal compression but sometimes fails to show the filling of the cyst.10 In this case, delayed CT-myelography can be helpful. Our diagnostic tool of choice is the MRI, which has great sensitivity and specificity for cerebrospinal fluid containing lesions. Cranially and caudally pushed epidural fat, displacement of the subarachnoid space and extension into the intervertebral foramen indicate an extradural lesion. With the MRI cord, atrophy and extent of myelomalacia can be assessed.10
Differential diagnosis to extradural arachnoid cysts are intradural arachnoid cysts, neurenteric cysts, perineural cysts, synovial cysts, meningocele, cystic neoplasms, congenital and traumatic epidermoid, dermoid, inflammatory cysts and cysticerosis.9
For patients with symptomatic arachnoid cysts, surgery is the treatment of choice.11 In this case, the cyst should be completely resected and the dural defect should be closed. To prevent recurrence or development of a liquorcele, thorough defect closure should be achieved.2 Puncture of the cystic wall, which can be done with CT direction, and aspiration of cystic fluid only result in temporary symptom relief. Passive and active fluid transport and the ball-valve mechanism rapidly allow the cyst to refill and the symptoms recur. Decompression causes prolonged symptom relief but the cyst grows further and becomes symptomatic once it reaches a considerable size. Wide decompression with complete resection of the dorsal stabilising ligaments causes instability with additional low back pain symptoms, which can hardly be distinguished from the pain caused by the cyst. To prevent instability-related complications as back pain, hypertrophy of scar tissue and early degeneration of discoligamental structures in our case treatment had to be extended to cyst resection and PLIF. So far there is no scientific evidence that any form of surgical decompression or fusion for degenerative lumbar spondylosis is effective when compared with natural history, placebo or conservative treatment,12 but a prospective long-term study has shown that a solid fusion (eg PLIF) improves the clinical outcome in patients undergoing decompression.13
The outcome of surgically treated patients with extradural spinal arachnoid cysts depends highly on the patient's age, the duration and degree of neurological damage.11, 14 Immediate pain relief after surgery is mostly reported but recurring back pain with and without radiation is common in long-term follow-ups (Figure 4). Alvisi et al11 reported poor long-term results in five of ten cases in a follow-up of 2–23 years eventhough the patients had fair to excellent symptom relief directly after surgery. The long-term results of our patient underline the importance to correctly diagnose and treat extradural spinal cysts. Postponed treatment caused pain chronification that had to be treated medically and psychosomatically. To help these patients, the early recognition is necessary and surgical treatment has to be improved. The widespread availability of MRI has greatly improved the diagnostic possibilities.
Long-term outcome after surgery on patients with extradural spinal arachnoid cysts. Review of the literature from 1993–2004. 12 cases. Mean follow-up after 20 months2, 5, 6, 9, 10, 15, 16 (excellent=no residual pain and complete relief of neurological deficits, good=mild residual pain but complete relief of neurological deficits, fair=persistent but slightly improved preoperative pain and neurological symptoms, poor=unchanged or worsened pain, unchanged neurological deficit)
References
Coffin CM et al. Posttraumatic spinal subarachnoid cysts. Eur Radiol 1996; 6: 523–525.
Stern Y, Spiegelmann R, Sadeh M . Spinal arachnoid cysts. Neurochirurgica 1991; 34: 127–130.
Sato K, Nagata K, Sugita Y . Spinal extradural meningeal cyst: correct radiological and histopathological diagnosis. Neurosurg Focus 2002; 13: 1–4.
Nabors MW et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68: 366–377.
Rohrer DC, Burchiel KJ, Gruber DP . Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation. J Neurosurg 78; 1993: 122–125.
Hatashita S, Kondo A, Shimizu T, Kurosu A, Ueno H . Spinal extradural achanoid cyst. Neurol Med Chir (Tokyo) 2001; 41: 318–321.
Rabb CH, McComb JG, Raffel C, Kennedy JG . Spinal arachnoid cysts in the pediatric age group: an association with neural tube defects. J Neurosurg 1992; 77: 369–372.
Fortuna A, Mercuri S . Intradural spinal cysts. Acta Neurochir (Wien) 1983; 68: 289–314.
Krings T, Lukas R, Reul J, Spetzger U, Reinges MHT, Gilsbach JM, Thron . Diagnostic and therapeutic management of spinal arachnoid cysts. Acta Neurochir (Wien) 2001; 143: 227–235.
Rimmelin A et al. Imaging of thoracic and lumbar spinal extradural cysts: report of two cases. Neuroradiology 1997; 39: 203–206.
Alvisi C, Cerisoli M, Giulioni M, Guerra L . Long-term results of surgically treated congenital intradural spinal arachnoid cysts. J Neurosurg 1987; 67: 333–335.
Gibson JN, Waddell G, Grant IC . Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev 2000; 3 CD001352.
Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS . Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine 2004; 29: 726–733.
Kendall BE, Valentine AR, Keis B . Spinal arachnoid cysts: clinical and radiological correlation with prognosis. Neuroradiology 1982; 22: 225–234.
Liu H et al. Large thoracolumbar extradural arachnoid cyst. Orthopedics 2004; 27: 225–226.
Ido K, Matsuoka H, Urushidani H . Effectiveness of a transforaminal surgical procedure for spinal extradural arachnoid cyst in the upper lumbar spine. J Clin Neuroscience 2002; 9: 694–696.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, Y., Reinke, M., Haschtmann, D. et al. Spinal extradural meningeal cyst with spinal stenosis. Spinal Cord 44, 457–460 (2006). https://doi.org/10.1038/sj.sc.3101848
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101848