Abstract
Study design:
Case–control study with pair-wise matching.
Objectives:
To investigate the level of locomotor performance in spinal cord injury (SCI) subjects.
Setting:
Spinal Cord Injury Center of Balgrist University Hospital in Zurich, Switzerland.
Methods:
Seven well-recovered SCI subjects and controls performed three tests: (1) 6-Min Walk Test (6MWT), (2) treadmill walking with full and restricted vision and (3) repetitive stepping over an obstacle. In the latter task, the subjects had to step over an obstacle as low as possible, without touching it. The subjects performed three blocks of 50 trials each. They received acoustic signals that indicated obstacle approach and foot clearance. During the third block, vision was restricted. The main outcome measures were: (1) distance walked, (2) leg muscle activity, joint kinematics and double-support duration, (3) foot clearance, number of obstacle hits, leg muscle activity, joint kinematics and swing phase duration.
Results:
(1) No difference in the 6MWT between the groups. (2) Independent from vision, the double-support duration was increased in SCI subjects. (3) SCI subjects learned generally at a slower rate. They touched the obstacle more frequently when vision became restricted. Furthermore, we observed differences between the groups in rectus femoris and tibialis anterior EMG activity and swing phase duration.
Conclusion:
It appears that even in well-recovered SCI subjects, small deficiencies in functional gait can be determined, which remain undetected by clinical walking tests. In addition, SCI subjects learned a locomotor task at a slower rate. This indicates that rehabilitation programs should train (adaptive) locomotion in different (sensory) environments.
Similar content being viewed by others
Introduction
Assessment of the recovery of functional walking is an important issue in the rehabilitation of subjects with a spinal cord injury (SCI). It is needed to evaluate therapeutical approaches such as body weight supported (automated) treadmill training1, 2, 3, 4, 5 to assess their effects on functional outcome.6, 7 Novel therapeutic approaches should also be evaluated at a functional level. In SCI, clinical assessment tools have been developed for evaluating qualitative aspects of gait (Walking Index for Spinal Cord Injury, WISCI)8, 9 or quantitative ones, such as timed walking tests.10 However, these clinical assessments concentrate on straight unobstructed walking and do not assess the possibility to adapt the gait pattern to the behavioral goals of the patient and the environmental constraints,11, 12, 13 which should be the final goal of rehabilitation.14 Indeed, it is still largely unknown to what extent patients with an SCI could adapt to external demands.15 Only a limited number of studies have investigated the locomotor capacity of patients with an SCI. It appeared that they walk with more attentional demand13 and use different kinematic strategies when stepping over obstacles14 or walking uphill.16 Furthermore, they can adapt gait speed, but only within a limited range and are unable to increase stride frequency.17 In addition, it is unknown whether SCI subjects experience differences in the acquisition of adaptive locomotor tasks during or after the rehabilitation program. Since improvement in motor performance strongly depends on feedback mechanisms, it could be expected that locomotor learning might be affected in SCI patients, since tactile and proprioceptive inputs are often impaired in SCI, as well as motor function.
Previously, Mulder and Geurts18 introduced a task set for recovery assessment, in which four categories were defined: (a) In the basic condition, simple straightforward gait on a flat level could be tested. (b) Cognitive manipulations tested noise conditions and dual-task performance during gait. (c) Perceptual manipulations investigated the effect of a reduction in the quality of visual information. (d) The introduction of sensorimotor manipulations should test the highest recovery stage (eg avoiding visible and unexpected obstacles).
The present pilot study tries to combine the two discussed issues: (1) can subjects with an SCI learn to adapt their walking pattern? and (2) can we determine the level of functional recovery in well-recovered SCI subjects as suggested by Mulder and Geurts?18 We used several experimental paradigms to investigate the level of locomotor performance (1) in the basic condition, (2) when influenced by a perceptual manipulation and, (3) when influenced by a sensorimotor manipulation. We hypothesized that (1) no differences exist between patients and healthy controls in unobstructed level walking, (2) slight differences exist when vision becomes restricted and (3) clear differences exist in learning and performing a sensorimotor manipulated task.
Methods
A case–control study with pair-wise matching was chosen to assess differences in level walking and in obstacle stepping between ambulatory SCI patients and healthy controls. The study was approved by the local Ethics Committee. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. All subjects were informed about the experiment and gave written consent.
Subjects
In Balgrist University Hospital, recovery of gait in SCI patients is documented using timed walking tests, such as the 6-Min Walk Test (6MWT). We used these data to select SCI subjects who had been admitted to the rehabilitation hospital at least 2 years ago and who had regained good walking function by the end of their rehabilitation program. Seven ASIA D patients were asked to participate in the experiment. They walked without the use of aids (WISCI II score of 20).8 The patients were matched for age and gender with a convenience sample of control subjects. Anamnesis revealed that, besides SCI in the patient group, all subjects had no orthopedic, neurological or cardiovascular diagnosis. One patient was taking antidepressant medication. Both groups consisted of five male and two female subjects. The SCI patients were on average 37.0±11.3 years old, 173.3±2.8 cm tall and weighed 67.9 kg. The time interval between SCI and the time of testing varied between 2 and 3 years. The controls were on average 37.1±12.4 years old, 179.4±7.4 cm tall and weighed 68.4±5.2 kg. Specific characteristics of the patients are presented in Table 1. Information about the lower extremity motor score (LEMS) at onset and end of rehabilitation was provided, as well as the duration of the rehabilitation. Impairments in proprioception were quantified by recording somatosensory-evoked potentials (SSEPs) of the tibial nerve.19 The SSEP latency provides information about the intactness of dorsal column tracts function that carries proprioceptive information. The SSEPs were made during the rehabilitation of the patients. However, SSEPs do not change significantly during the course after traumatic SCI.19
Experimental design
In the basic condition, normal level walking was investigated by the assessment of a 6MWT. This test was performed after the subjects had completed all other experiments. This was performed to investigate whether fatigue had influenced the task performance of the other tests. All subjects were instructed to walk at a normal speed. The 6MWT has a strong correlation with cardiovascular endurance20, 21 and has been investigated for its validity and reliability recently in SCI patients.10
Perceptual manipulations were investigated when walking on a treadmill. Before the experiment started, all subjects walked for 5 min on the treadmill at a speed of 0.69 ms−1 to become familiar with treadmill walking. After this, they had to walk with full and restricted vision on the treadmill. This was repeated after the obstacle experiment (see next paragraph) to investigate whether fatigue might have influenced differences in performance between SCI subjects and the controls. Restricted vision was achieved by wearing special glasses, which covered the visual field about 5° below the horizontal level. In this way, the subjects received no visual input about the treadmill and the legs.
Sensorimotor manipulations were investigated by means of an obstacle experiment.22, 23 The subjects had to learn to step repetitively over the obstacle, as low as possible, without touching it. They performed three blocks, each consisting of 50 steps over the obstacle. There was a break of 5 min between successive blocks. In the first two blocks, the subjects stepped over the obstacle with full vision. In the third block, they had to perform the same task with restricted vision, wearing the same glasses as described above. During the whole experiment, the subjects wore their training shoes and a safety suspension vest was available for all subjects at request.
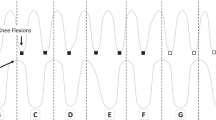
In general, the subjects walked on a split-belt treadmill (Woodway, Weil am Rhein, Germany) with a speed of 0.69 ms−1. They were allowed to have hand contact with the parallel bars beside the treadmill. A custom-built obstacle-machine was placed next to the treadmill in order to study repetitive stepping over the obstacle (Figure 1a). The obstacle consisted of a foam stick, 11 cm above the treadmill. It was attached to the obstacle machine in such a way that it folded back by slight touch and caused no stumbling. Eight one-dimensional force sensors were located underneath the treadmill belts, one under each corner of each split-belt (Figure 1a). The force sensors underneath the right treadmill belt were used to indicate heel strike (HS1) of the right foot. HS1 started the movement of the obstacle (Figure 1b) and the recording of various experimental measures. After release, the obstacle moved with the same speed as the treadmill and the subject could step with the right foot over the obstacle without changing the rhythm of walking cadence. A short beep of 100 ms duration, which was provided at the middle of the stance phase (33% of the step cycle), served as a warning signal and indicated the approaching obstacle. After the subject stepped over the obstacle, the obstacle folded up at the end of the treadmill and moved back to its starting position. The time between obstacle steps varied randomly between 9 and 16 s, or 6–11 normal step cycles.
Experimental setup. (a) The obstacle moves with the same speed as the treadmill. When it reaches the end of the treadmill, it folds up and moves back to its starting position. The light-sensitive diodes record foot clearance over the obstacle. (b) Timing of all events during a single step over the obstacle and time periods during which the measures were analyzed. Abbreviations: HS, heel strike before (HS1) or after (HS2) stepping over the obstacle; TO, toe off before (TO1) or after (TO2) stepping over the obstacle; AWS, acoustic warning signal indicating the approaching obstacle; AFS, acoustic feedback signal indicating the level of foot clearance
Data recording
The 6MWT could easily be assessed using a stopwatch and distance covered in the 6 min.
In the experiments that involved treadmill walking, the following measures were recorded: (a) leg muscle EMG signals, (b) joint movements, (c) vertical forces exerted by the subject on the treadmill, and in the obstacle experiment, (d) distance between the foot and the obstacle and (e) number of obstacle hits. (a) EMG signals of four muscles of the right leg were recorded using surface electrodes: the rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA) and gastrocnemius medialis (GM) muscles. The EMG signals were amplified, band-pass filtered (30–300 Hz) and transferred together with the biomechanical signals to a PC using Soleasy software (ALEA Solutions GmbH, Zurich, Switzerland) via an analog-to-digital converter. All signals were sampled at 1000 Hz. (b) Ankle, knee and hip joint movements of the right leg were monitored using electrogoniometers (Biometrics Ltd, Gwent, UK) fixed at the lateral aspect of each joint. (c) The vertical forces measured by the force sensors underneath the right treadmill belt were used to determine HS1, toe off before stepping over the obstacle (TO1), heel strike after stepping over the obstacle (HS2) and second toe off (TO2). (d) The level of foot clearance was determined by a vertical linear array of eight light-sensitive diodes, which were attached to the obstacle machine above the foam stick (Figure 1a). The lowest diode was placed 2 cm above the obstacle and the distance between each diode was 2 cm (3 cm for the upper two light sensitive diodes). The signal of the lowest activated diode was recorded for each step over the obstacle. Furthermore, the lowest activated diode provided an acoustic feedback signal about foot clearance to the subject. Greater foot clearance was signaled by a higher acoustic feedback signal. When the lowest diode was activated (optimal foot clearance), a double-beep signal (707 and 1400 Hz sinusoidal signal, 600 ms duration) was heard. The other feedback signals consisted of single beeps (125, 176, 250, 354, 500, 707 and 1000 Hz rectangular signal of 400 ms duration for the second lowest to the highest diode, respectively). (e) The number of obstacle hits was also recorded by the obstacle machine.
Data analysis
For assessing the effect of perceptual manipulation, 20 recorded step cycles were normalized to 1000 samples and averaged to one step cycle. Three parameters were determined: (1) double-support duration as a percentage of the normalized step cycle, (2) the strength of leg muscle activation as determined by the root mean square (RMS) of the rectified EMG signals and, (3) the range of motion (ROM) of the joint angles trajectories. Four different conditions were measured: before and after the obstacle experiment with full and restricted vision. To make the comparison between SCI patients and controls, the RMS and ROM values were normalized and expressed as percentages of the value measured before the obstacle experiment with full vision.
Sensorimotor manipulations were tested during the obstacle experiment. The EMG signals were rectified and for each step over the obstacle, the RMS was determined for an interval between TO1 and TO2 (Figure 1b). EMG recordings containing movement artefacts were removed from the analysis. Indeed, BF activity of one control subject could not be analyzed throughout the experiment. For the interval between TO1 and HS2, the ROM for each joint movement signal was calculated (Figure 1b). The duration of the swing phase was determined by assessing the time interval between TO1 and HS2 using the ground reaction forces (Figure 1b). Finally, foot clearance height was measured using the light-sensitive diodes. When an obstacle hit occurred, the foot clearance measurement was removed from the data analysis. Only obstacle hits that occurred between TO1 and HS2 were included in the analysis.
In general, the subject's performance improved when the measures decreased,22 that is, (1) a lower level of foot clearance over the obstacle, (2) a decrease in number of obstacle hits, (3) smaller RMS values for leg muscle activation, (4) decreased ROMs of lower limb joints, and/or (5) a shortening of the swing phase duration over the obstacle.
Each block consisted of 50 steps over the obstacle. For every five steps over the obstacle, we calculated the average value of each measure for each subject. In each subject, each measure (except for foot clearance) was expressed as a percentage of the average value calculated over the first five steps over the obstacle. For easier interpretation, we calculated grand mean values with standard deviations (SD) of the normalized values (except for foot clearance) for each block of trials and for each measure.
Statistical analysis
Basic condition
Differences in performance of the 6MWT were analyzed by a Wilcoxon ranked sum test.
Perceptual manipulations
Differences in double support, RMS and ROM were analyzed by analysis of variance (ANOVA) for repeated measures with four levels of the within-subject factor (level walking with full and restricted vision before and after the obstacle experiment) and two levels of the between-subjects factor (SCI patients versus controls). Pair-wise comparisons were adjusted by the Bonferroni correction. Perceptual manipulations were tested for six SCI and six control subjects.
Sensorimotor manipulations
To determine the differences in the performance between the groups and the blocks, we used the normalized values of the blocks in an ANOVA for repeated measures with subsequent Bonferroni's correction for pair-wise comparisons. We determined differences between the groups (two levels: healthy subjects and SCI patients), the three blocks and the interaction (groups × blocks). Differences in the number of obstacle hits were also tested using ANOVA for repeated measures with subsequent Bonferroni's correction for pair-wise comparisons.
In addition, the rate of adaptive learning was assessed from the change in foot clearance, by fitting a power function24 to the foot clearance data. One characteristic of a power function is that logarithmic transformation of both the number of trials and the performance measure result in a linear relationship. In the obtained linear regression equation (y=b0+b1 × x), the regression coefficient b1 quantifies the adaptive rate. For all analyses, α was set at 0.05.
Results
Basic condition
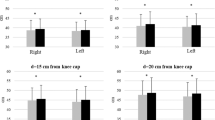
The SCI patients covered an average distance of 524 m (SD=56 m), and the controls covered 554 m (SD=40 m) in 6 min (Figure 2a). This difference was not significant (P=0.25).
Locomotor characteristics: covered distance and double-support duration. (a) Box and whisker plot of the covered distance in the 6-Min Walk Test (6MWT) of seven spinal cord injured (SCI) and seven healthy subjects. The lower and upper 25% of the observations are presented as lines (whiskers) below and above the box, respectively. The box contains the middle 50% of the observations and is divided into two by the median (50th percentile). (b) Average double-support duration (with standard deviation) with full and restricted vision expressed as the percentage of the step cycle duration during normal treadmill walking. The recordings were made before and after the obstacle stepping experiment was performed. *Double-support duration was longer in SCI compared to healthy subjects (P<0.05)
Perceptual manipulations
Figure 2b shows that during treadmill walking, patients had a significantly longer double-support duration (% of step cycle duration) compared to the controls (F(1,10)=8.62, P=0.015). There was no difference observed for condition or interaction.
Table 2 shows the leg muscle activity and joint ROM recorded before and after the obstacle experiment with full and restricted vision. The measures are expressed as a percentage of the value obtained during the condition of treadmill walking with full vision, before the obstacle experiment was started. There was no difference observed for muscle activity and joint range of motion.
Sensorimotor manipulations
The number of obstacle hits differed between the blocks (F(2,18)=9.11, P=0.002). SCI subjects touched the obstacle significantly more when vision became restricted (mean=12.3 hits, SD=11.7, range 2–33), compared to the first block (mean=0.83 hits, SD=0.75, range 0–2, P=0.011) and second block (mean=0.5 hits, SD=0.83, range 0–2, P=0.008). The controls touched the obstacle also more frequently in the third block (mean=5.0 hits, SD=2.55, range 2–8), compared to the first (mean=0.6 hits, SD=1.34, range 0–3) and second (mean=1.4 hits, SD=0.89, range 0–2) blocks. However, this difference was not significant.
We correlated the number of obstacle hits in the third block, as a measure of complex adaptive locomotor task performance with the double-support duration recorded during walking with full and restricted vision before the obstacle experiment. We could analyze the data of only four control and five SCI subjects. For all subjects, Spearman's correlation coefficient (ρ) was 0.32 (not significant, NS; full vision) and 0.68 (P=0.04; restricted vision). If both groups were analyzed separately, ρ was 0.40 (NS; full and restricted vision) in the controls, but in the patients it accounted to −0.10 (NS; full vision) and 0.70 (NS; restricted vision).
Adaptive rate
Figure 3 shows the averaged foot clearance values for each block of five trials for the healthy (Figure 3A) and SCI (Figure 3B) subjects. One SCI subject hit the obstacle 33 times in the third block. We removed these data from the analysis of the foot clearance, since 17 data points were considered too few, to analyze. To obtain equal power, we removed also the data of the matched control subject for the third block. For the healthy subjects, the adaptive rate was significant for both the full and restricted vision conditions, with regression coefficients being −0.172 (SE=0.033; P<0.001) for the first, −0.089 (SE=0.025; P<0.001) for the second and −0.403 (SE=0.046; P<0.001) for the third block. For the subjects with an SCI, the adaptive rate was not significant even for the more difficult condition of restricted vision, with regression coefficients being −0.058 (SE=0.048; P=0.23) for the first, −0.056 (SE=0.028; P=0.053) for the second and −0.073 (SE=0.064; P=0.26) for the third block.
Course of the foot clearance during the obstacle avoidance task. Scatter-plots of the foot clearance for (A) the healthy and (B) SCI subjects during blocks (a) 1, (b) 2 and (c) 3. Each data point represents the average of five steps over the obstacle for each subject. The adaptive rate of the fitted power curve is presented as b1 (regression coefficient). *Significant regression coefficient
The grand mean values and SD of the performance measures are shown in Table 3. ANOVA for repeated measures showed that the foot clearance performance differed between the blocks (F(2,22)=24.87; P<0.001), but not between the groups. For the healthy subjects, the foot clearance was higher in block 3 compared to block 1 (P<0.05). For both groups, the foot clearance was higher in the third compared to the second block (healthy: P<0.001; SCI: P<0.01).
Figure 4A shows the course of the RF EMG activity during the experiment. RF EMG activity differed between the groups (F(1,12)=11.17; P<0.01), the blocks (F(2,24)=43.57; P<0.001) and its interaction (F(2,24)=8.11; P<0.01). RF EMG activity was lower for the SCI subjects compared to the healthy subjects during block 2 (P<0.01) and a trend towards a difference during block 3 (P=0.08). For the healthy subjects, RF activity was higher during block 3 compared to blocks 1 and 2 (for both: P<0.001). For the SCI subjects, RF activity was less during block 2 compared to blocks 1 and 3 (for both: P<0.001). BF activity differed between the blocks (F(2,22)=9.94; P<0.001). For the healthy subjects, BF activity was less during block 2 compared to blocks 1 and 3 (for both: P<0.05). Figure 4B shows the course of the TA EMG activity during the experiment. TA activity differed significantly between the blocks (F(2,24)=29.85; P<0.001) and its interaction was also significant (F(2,24)=19.01; P<0.001). TA EMG activity was lower for the SCI subjects compared to the healthy subjects during block 3 (P<0.05). Furthermore, for the healthy subjects, TA EMG activity was greater during block 3 compared to blocks 1 and 2 (for both: P<0.001). In addition, TA activity was higher during block 1 compared to block 2 (P<0.01). GM EMG activity differed between the blocks (F(2,24)=4.39; P<0.05).
Time course of RF and TA EMG activity and the swing phase duration. Scatter-plots of normalized (A) RF muscle activity, (B) TA muscle activity and (C) swing phase duration for the (a) healthy and (b) SCI subjects. Gray areas present differences between the two groups in congruent blocks. Each data point represents the average of five steps over the obstacle for each subject. The measures were expressed as a percentage of the average value calculated over the first five steps over the obstacle. Note the different scales of the y-axis
The hip ROM differed significantly between the blocks (F(2,24)=35.54; P<0.001) and its interaction was significant (F(2,24)=33.55; P<0.001). For the healthy subjects, hip ROM was increased during block 3 compared to blocks 1 and 2 (for both: P<0.001). The knee ROM differed between the blocks (F(2,24)=37.94; P<0.001) and the interaction was significant (F(2,24)=11.60; P<0.001). The knee ROM was increased in block 3 compared to blocks 1 and 2 (for both: P<0.001). For the ankle ROM, only the interaction was significant (F(2,24)=4.24; P<0.05). For the SCI subjects, hip, knee and ankle ROM did not differ between the blocks of trials.
The duration of the swing phase (Figure 4c) was significantly different between the groups (F(1,12)=10.21; P<0.01) and the blocks (F(2,24)=41.04; P<0.001). Healthy subjects had a longer swing phase duration compared to SCI subjects during block 2 (P<0.05). Although the healthy subjects increased their foot clearance at onset of block 3, which did not occur in the SCI subjects, the difference was not significant (P=0.11). Furthermore, for the healthy subjects the swing phase duration was shorter in block 1 compared to blocks 2 (P<0.01) and 3 (P<0.001). For the SCI subjects, swing phase duration was longer during block 3 compared to blocks 1 and 2 (for both: P<0.001).
Discussion
The aim of the present study was to assess locomotor performance and the capacity for adaptive learning in well-recovered SCI individuals during gait challenges of different complexities. The study was based on tasks that represent different categories of recovery.18 Owing to the small number of patients tested, the power of this study is limited, which makes it difficult to generalize findings to the population of subjects with an SCI. However, it presents some interesting findings that are worth further investigation in future studies.
The present findings are only partially in line with the temporal order of recovery after a lesion of the central nervous system as proposed by Mulder and Geurts.18 According to this study, performance under simple conditions should improve first, followed by a gradual decrease in cognitive regulation and visual dependency. Finally, the reaction and anticipation on external disturbances, such as obstacles, should become normalized.
The first hypothesis could be partially accepted. In the basic condition, the healthy and SCI subjects showed no difference in distance covered during the 6 min of walking. The walking speed of both groups could be considered normal to high when compared to earlier studies,25, 26, 27 moreover since in the present study the 6MWT was applied after all other tests were performed. However, a more detailed analysis of normal treadmill walking revealed that the well-recovered SCI subjects had longer double-support duration, which indicates that the basic function had not been fully normalized. This occurred already at a slow walking speed (0.69 ms−1). At higher walking speeds, larger differences in double stance duration between the healthy and SCI subjects might be expected. An increase in double-support duration is in line with a recent study.28 During double support, there are smaller balance and muscle strength requirements than during the single support phase and thus double support enhances body stabilization.29 In patients in early stages of multiple sclerosis, a prolonged double-support phase indicated a motor abnormality prior to any functional disturbance.30 Increased double-support duration is also found in patients with slight knee arthrosis31 and in patients recovering from stroke, when a cognitive task is applied.32
Although hand contact might have reduced the balance requirements during single support in the present study, it should not have influenced the percentage of stride cycle spent in double support.33 Furthermore, double-support duration measured during walking with restricted vision appears to be strongly correlated with the performance of the more complex locomotor task. Therefore, the assessment of double-support duration with restricted vision could be of clinical interest to determine locomotor deficits in SCI subjects.
With respect to the second hypothesis, the SCI subjects in the present study already passed the second stage according to Mulder and Geurts.18 The perceptual manipulation experiment with restricted vision had no influence on level walking on a treadmill.
With respect to the third hypothesis, there were some differences between the two groups in the acquisition and performance of the sensorimotor manipulated task, that is, repetitive stepping over an obstacle. The goal of the task was to minimize foot clearance without touching the obstacle, that is, to perform a high-precision task. While foot clearance in SCI subjects did not show a similar adaptation as able-bodied subjects did, we suggest that the well-recovered SCI subjects indeed could improve their performance; however, with higher variability. This might indicate that sensory impairment indeed affects the process of motor learning in these subjects negatively. In addition, the number of obstacle hits increased in both groups when vision became restricted, but only significantly in the SCI subjects. In this condition, the subjects needed to integrate the acoustic (foot clearance) and tactile (obstacle hit) feedback, while proprioceptive and tactile information were used to control the leg movements. Of the three SCI subjects who most frequently hit the obstacle, two had pathological SSEPs. Therefore, the poorer performance could be due to defective proprioceptive and tactile mechanisms.
The poorer performance could also partially be explained by the high balance requirement of the task. The slow walking speed, which was used in the experiment, increases the time needed to step over the obstacle. This implies longer single support duration of the trailing leg, which would increase balance and strength requirements. Impaired stability of this leg might have affected the performance of the leading leg negatively. Indeed, the SCI subjects who touched the obstacle most frequently had some weakness of extensor muscles (triceps surae and/or gluteal muscles) as assessed with manual muscle testing, which are important in maintaining balance.34, 35, 36 However, relative small deficits in muscle strength could not be determined using manual muscle testing. Furthermore, even in healthy subjects, an increase in attentional demand evolves in parallel to an increase in balance requirements during walking.29 For SCI subjects, walking required more attention than for healthy subjects13 and requires more working memory capacity, which might be responsible for the poorer task performance.
In general, muscle activity decreased during blocks 1 and 2, which indicates that the task was performed more efficiently.22 However, it appeared that the normalized RF and TA muscle activity were higher during blocks 2 and 3 in healthy compared to SCI subjects. This might be explained by the normalization method. Healthy subjects might have performed the task initially with less muscle activity. Then, at onset of the third block, they stepped higher over the obstacle, which is associated by a longer travel path37 and required more muscle EMG activity. In contrast, already at onset of the experiment, the SCI subjects might have performed the task with relative high muscle activity. Although the foot clearance increased at onset of the third block, they did not largely increase muscle activity nor swing phase duration, which might partially explain the increase in obstacle hits in the third block of the SCI subjects.
SCI and age
The oldest SCI subject (59 years) touched the obstacle 33 times during the third block with restricted vision. In addition, we found a strong linear relationship between the age of the SCI subjects and the number of obstacle hits they made in the third block (r=0.87, P<0.01). This relationship was less strong for the control subjects (r=0.51, not significant). This suggests that the combination of age and SCI strongly influences locomotor performance. Indeed, the recovery of locomotor function in SCI subjects with ASIA C depends on age.38, 39 A previous study that investigated the same obstacle task in healthy young and elderly subjects found that the elderly touched the obstacle significantly more compared to the young ones and were not able to reoptimize foot clearance with restricted vision.40 It is suggested that the combination of age and motor impairment, rather than each factor separately, affects the acquisition and performance of a locomotor task, especially with restricted vision. However, future studies are needed to substantiate this issue.
Methodological considerations
Sensory impairment in the dermatomes of the foot (L4–S1) in combination with wearing shoes, might have led to a reduced perception of obstacle hits in the SCI subjects. Unawareness of an obstacle hit would clearly not lead to an improvement in task performance, especially during block 3. However, we expect that it is unlikely that this accounted for the poorer performance during the obstacle experiment. Mainly three subjects touched the obstacle more frequently and this occurred randomly during the block, which indicates that they perceived the obstacle hit and adapted the leg kinematics accordingly to increase foot clearance. It was unlikely that fatigue caused the poorer performance of the SCI subjects during the obstacle experiment, since the 6MWT (performed after all other tests were finished) did not differ between the groups and the walking speed was according to the literature,25, 26, 27 normal to high.
Conclusions
In conclusion, at first sight, there appears to be no difference in walking function in these well-recovered SCI subjects, when assessed with a clinical test. A more detailed analysis shows, however, that these SCI subjects require a longer double-support duration and have greater difficulty in performing, but especially in acquiring, a new adaptive locomotor task. We suggest that rehabilitation programs should focus more on practicing locomotor skills under conditions with reduced sensory inputs, such as restricted vision. In addition, such programs should also train patients in adapting their gait pattern to external demands such as avoiding obstacles. The relatively simple measure of double-support duration analyzed during walking with restricted vision was strongly correlated to the complex adaptive locomotor performance. Future studies might focus more on the use of this measure as a clinical tool to predict locomotor performance in patients with a motor deficit or as a follow-up measure to assess improvement in walking function.
References
Colombo G, Joerg M, Schreier R, Dietz V . Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev 2000; 37: 693–700.
Colombo G, Wirz M, Dietz V . Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord 2001; 39: 252–255.
Dietz V, Colombo G, Jensen L . Locomotor activity in spinal man. Lancet 1994; 344: 1260–1263.
Hesse S, Schmidt H, Werner C, Bardeleben A . Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol 2003; 16: 705–710.
Wernig A, Muller S . Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia 1992; 30: 229–238.
Dietz V . Spinal cord lesion: effects of and perspectives for treatment. Neural Plast 2001; 8: 83–90.
Wirz M, Colombo G, Dietz V . Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatry 2001; 71: 93–96.
Dittuno PL, Dittuno Jr JF . Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord 2001; 39: 654–656.
Ditunno Jr JF et al. Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord 2000; 38: 234–243.
Van Hedel HJ, Wirz M, Dietz V . Assessing walking ability in subjects with a spinal cord injury: validity and reliability of walking tests. Arch Phys Med Rehabil 2005; 86: 190–196.
Forssberg H . Spinal locomotor functions and descending control. In: Sjölund B, Björklund A (eds). Brain Stem Control of Spinal Mechanisms. Elsevier Biomedical Press: Amsterdam 1982, pp 253–271.
Ladouceur M, Pepin A, Norman KE, Barbeau H . Recovery of walking after spinal cord injury. Adv Neurol 1997; 72: 249–255.
Lajoie Y, Barbeau H, Hamelin M . Attentional requirements of walking in spinal cord injured patients compared to normal subjects. Spinal Cord 1999; 37: 245–250.
Ladouceur M, Barbeau H, McFadyen BJ . Kinematic adaptations of spinal cord-injured subjects during obstructed walking. Neurorehabil Neural Repair 2003; 17: 25–31.
Barbeau H, Fung J, Leroux A, Ladouceur M . A review of the adaptability and recovery of locomotion after spinal cord injury. Prog Brain Res 2002; 137: 9–25.
Leroux A, Fung J, Barbeau H . Adaption of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp Brain Res 1999; 126: 359–368.
Pepin A, Norman KE, Barbeau H . Treadmill walking in incomplete spinal-cord-injured subjects: 1. Adaptation to changes in speed. Spinal Cord 2003; 41: 257–270.
Mulder T, Geurts A . Recovery of motor skill following nervous system disorders: a behavioral emphasis. Baillieres Clin Neurol 1993; 2: 1–13.
Curt A, Dietz V . Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocol in predicting outcome. Arch Phys Med Rehabil 1997; 78: 39–43.
Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM . Two-, six-, and 12-min walking tests in respiratory disease. BMJ (Clin Res Ed) 1982; 284: 1607–1608.
Guyatt GH et al. The 6-min walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–923.
Erni T, Dietz V . Obstacle avoidance during human walking: learning rate and cross-modal transfer. J Physiol 2001; 534: 303–312.
Van Hedel HJ, Biedermann M, Erni T, Dietz V . Obstacle avoidance during human walking: transfer of motor skill from one leg to the other. J Physiol 2002; 543: 709–717.
Snoddy GS . Learning and stability. J Appl Psychol 1926; 10: 1–36.
Bohannon RW . Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing 1997; 26: 15–19.
Steffen TM, Hacker TA, Mollinger L . Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 2002; 82: 128–137.
Zijlstra W . Assessment of spatio-temporal parameters during unconstrained walking. Eur J Appl Physiol 2004; 92: 39–44.
Pepin A, Ladouceur M, Barbeau H . Treadmill walking in incomplete spinal-cord-injured subjects: 2. Factors limiting the maximal speed. Spinal Cord 2003; 41: 271–279.
Lajoie Y, Teasdale N, Bard C, Fleury M . Attentional demands for static and dynamic equilibrium. Exp Brain Res 1993; 97: 139–144.
Benedetti MG, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S . Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999; 5: 363–368.
Gok H, Ergin S, Yavuzer G . Kinetic and kinematic characteristics of gait in patients with medial knee arthrosis. Acta Orthop Scand 2002; 73: 647–652.
Bowen A, Wenman R, Mickelborough J, Foster J, Hill E, Tallis R . Dual-task effects of talking while walking on velocity and balance following a stroke. Age Ageing 2001; 30: 319–323.
Siler WL, Jorgensen AL, Norris RA . Grasping the handrails during treadmill walking does not alter sagittal plane kinematics of walking. Arch Phys Med Rehabil 1997; 78: 393–398.
Winter DA . The Biomechanics and Motor Control of Human Gait: Normal Elderly and Pathological 2nd edn. Graphic Services, University of Waterloo: Ontario 1991.
Winter DA, MacKinnon CD, Ruder GK, Wieman C . An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res 1993; 97: 359–367.
Yang JF, Stein RB, James KB . Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 1991; 87: 679–687.
Patla AE, Rietdyk S . Visual control of limb trajectory over obstacles during locomotion: effect of obstacle height and width. Gait Posture 1993; 1: 45–60.
Burns SP, Golding DG, Rolle Jr WA, Graziani V, Ditunno Jr JF . Recovery of ambulation in motor-incomplete tetraplegia. Arch Phys Med Rehabil 1997; 78: 1169–1172.
Waters RL, Adkins RH, Yakura JS, Sie I . Motor and sensory recovery following incomplete paraplegia. Arch Phys Med Rehabil 1994; 75: 67–72.
Van Hedel HJ, Dietz V . The influence of age on learning a locomotor task. Clin Neurophysiol 2004; 115: 2134–2143.
Acknowledgements
We are grateful to all the subjects who participated in this study. Furthermore, we thank T Lam for her English language corrections. This research was supported by the Swiss National Science Foundation (NCCR on Neuronal Plasticity and Repair).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Hedel, H., Wirth, B. & Dietz, V. Limits of locomotor ability in subjects with a spinal cord injury. Spinal Cord 43, 593–603 (2005). https://doi.org/10.1038/sj.sc.3101768
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101768
Keywords
This article is cited by
-
Autogenic biofeedback training improves autonomic responses in a participant with cervical motor complete spinal cord injury- case report
Spinal Cord Series and Cases (2023)
-
Properties of the surface electromyogram following traumatic spinal cord injury: a scoping review
Journal of NeuroEngineering and Rehabilitation (2021)
-
Gait variability following abrupt removal of external stabilization decreases with practice in incomplete spinal cord injury but increases in non-impaired individuals
Journal of NeuroEngineering and Rehabilitation (2019)