Abstract
Study design: Case report with a review of scientific literature.
Objective: To describe the course of tuberculous spinal disease (Pott's disease) complicated by pyogenic and tuberculous empyema, and chylothorax as there has been an increase in the numbers of notified cases of tuberculosis in the UK1. To the best of our knowledge, a similar case has not been reported previously in the UK, although there has been a report of bilateral chylothorax associated with Pott's disease.
Setting: A national spinal injuries unit in a Scottish university teaching hospital.
Methods: Review of literature on the chemotherapy of spinal tuberculosis and the role of streptokinase in the treatment of empyema and the relation between spinal tuberculosis, empyema and chylothorax.
Results: Although spinal tuberculosis was recognised and treated appropriately with chemotherapy, the patient sustained pleural involvement with later development of both empyema and chylothorax.
Conclusion: The case highlights the difficulties in the treatment of tuberculosis of the spine inspite of the presence of fully sensitive organisms and early institution of appropriate chemotherapy. In the absence of surgical debridement, the duration and dosage of chemotherapy as practised in the initial period may have to be prolonged into the continuation phase. The thoracic duct can be damaged either because of extension of the tuberculosis itself or because of instillation of intrapleural streptokinase for treatment of pleural empyema leading to chylothorax. There is a need for randomised trials of intrapleural streptokinase treatment in tuberculous empyema.
Similar content being viewed by others
Case report
A 67-year-old Caucasian male was admitted to our hospital having fallen at home (where he lived alone) 3 days previously. He was doubly incontinent and unable to walk. The fall was preceded by 3 months of malaise. There was no history of travel abroad or of contact with anyone with tuberculosis. He smoked 10 cigars daily and his weekly alcohol intake was at least 60 units.
On admission, he had incomplete L2 paraplegia and a large full-thickness sacral sore.
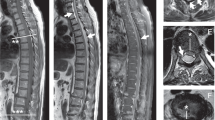
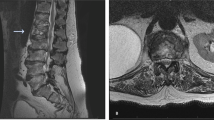
His weight was estimated to be 50 kg. Routine haematological and biochemical tests were normal apart from a ESR of 76 mm/h. Plain radiograph of thoracic spine showed bony destruction of the 12th thoracic vertebra. MRI spine confirmed abnormal soft tissue signal centred at T12/L1 intervertebral disc with compression of the conus by extradural soft tissue mass. Axial CT showed a paravertebral abscess abutting against the diaphragm (Figure 1). CT-guided biopsy of the paravertebral abscess showed numerous alcohol and acid-fast bacilli and grew Mycobacterium tuberculosis sensitive to isoniazid (INH), rifampicin, pyrazinamide and ethambutol. He was sputum smear negative.
He was not fit for surgical debridement of the abscess. He was commenced on INH 300 mg, rifampicin 450 mg, pyrazinamide 1.5 gm and 10 mg of pyridoxine daily. He was nursed on an air fluidised mattress. The sacral sore was debrided and treated with topical dressings and pressure closure system. After good clinical response with increase in appetite and reduction of malaise, the pyrazinamide was stopped after 8 weeks. Over the next week, the patient became very unwell, pyrexial and tachypnoeic with signs of a left-sided pleural effusion.
Repeat CT thorax revealed pulmonary consolidation with large left pleural effusion communicating with left paravertebral abscess through the diaphragm (Figure 2). Closed chest drainage yielded purulent material containing Gram-positive cocci, a moderate amount of WBC (60% polymorphs and 40% lymphocytes) and a profuse amount of acid-fast bacilli which, on repeat culture, again grew fully sensitive M. tuberculosis as before. Pyrazinamide was restarted with daily instillation of streptokinase (250000 units in 40 ml of saline) intrapleurally for 10 days. A course of intravenous ceftriaxone 2 gm/day and gentamycin 120 mg BD daily was also given and oral Prednisolone (30 mg daily) started. The chest drained a total of 4 l of frank pus over the next 10 days. The fluid then turned milky white and on analysis a triglyceride content >110 mg/dl confirmed a chylothorax.
The chylothorax was treated conservatively with a low fat diet consisting of medium chain triglyceride and the chylous discharge abated. The chest drain continues to drain several milliliters of purulent material daily. The patient's clinical condition has improved, but he remains paraplegic.
Discussion
Our patient had clinical and imaging evidence of tuberculosis, positive biopsy smear for acid-fast bacilli and positive culture for fully sensitive M. tuberculosis. Surgical debridement of the paraspinal abscess was not carried out as the patient's general condition was poor. Without doubt, antituberculous drugs remain the cornerstone of treatment as they control the morbidity and improve the prognosis for spinal tuberculosis. The actual duration and content of antituberculous chemotherapy, however, remains controversial. The initial treatment with four drug regimens is recommended by the World Health Organization.2 The Joint Tuberculous Committee of the British Thoracic Society3 also advocates four drug initial regimens with the proviso that the fourth drug (ethambutol) be omitted in patients with low risk of resistance to isoniazid; that is, previously untreated white patients who are known to be HIV negative or thought likely to be HIV negative on risk assessment (as was our case), and who are not contacts of another patient with known drug-resistant organisms. The recommendations agree that a three-drug regimen can be used in those with fully sensitive organisms, and also stress the need for ascertaining bacteriological confirmation and drug susceptibility of the organism, whenever possible, as was done in the case of our patient via biopsy of the paravertebral abscess, which revealed fully sensitive M. tuberculosis. He was accordingly given treatment with three drugs in the first 2 months as per the recommendations of the Joint Committee of the British Thoracic Society guidelines for treatment of tuberculosis in the UK.3 Professor Maung-Sang Moon in his recent review4 also acknowledges that newer chemotherapy regimens with four drugs has its advantages, but does not necessarily produce a satisfactory outcome in all cases. During the subsequent continuation phase of chemotherapy pyrazinamide was initially stopped, but was restarted because of the discovery of the presence of viable M. tuberculosis in the empyema fluid 8 weeks after starting the treatment. At that stage, the patient also had evidence of a superadded pyogenic empyema as evidenced by clinical deterioration with pyrexia, tachypnoea and signs of a left-sided pleural collection confirmed by the CT scan.
The empyema appears to have arisen because of erosion through the diaphragm. Extension of a paraspinal abscess to the psoas is well recognised,5 but we are not aware of diaphragmatic rupture as a complication of tuberculosis with consequent pleural abscess extension. The Gram-positive cocci and high neutrophil count may suggest a contributing bacterial pneumonia necessitating a course of intravenous antibiotics with good response.
Thoracic empyemas continue to cause substantial morbidity and mortality despite the advent of antimicrobial therapy.6 Conventional first-line treatment consisting of intercostal tube drainage may fail in the presence of fibrin deposition and/or loculation in the pleural space. While surgery including thoracotomy and decortication remains the definitive treatment, in these cases enzymatic debridement of the pleural cavity with streptokinase is a noninvasive option first described by Tillet and Sherry7 in 1949. Its use did not gain widespread acceptance because of reports of rare occurrence of intrapleural haemorrhage and systemic fibrinolysis.8 With the availability of purer fibrinolytic preparations there has been a resurgence in the use of intrapleural fibrinolytic therapy.9 In a recent randomised trial, intrapleural streptokinase improves the drainage of infected material in community-acquired pneumonias.10 Whether intrapleural streptokinase also improves clinical outcomes in pleural infections other than that because of community-acquired pneumonias needs to be established in randomised trials. However, streptokinase has a definite role in the treatment of empyema as a debriding agent11 and was used on that basis in our patient who was in any case not fit for surgery. The development of chylothorax is intriguing. Chylothorax is known to result from the obstruction of the thoracic duct usually by a diffuse process such as an infiltrating malignancy, mediastinal fibrosis or a granulomatous disorder-like tuberculosis. In a 1935 review of 55 patients with nontraumatic chylothorax, Yater12 found only 11 cases due to tuberculosis, but none of these 11 patients had tuberculous spondylitis. While Richard Menzies and Hidvegi13 have described a patient with Pott's disease and chylothorax, their patient had a past history of untreated tuberculous infection, presented with bilateral chylothoraces, was subsequently diagnosed to have tuberculous spondylitis and responded to antituberculous chemotherapy with three agents (isoniazid, rifampicin and ethambutol). Our patient, on the other hand, had no previous contact with tuberculosis, presented with Pott's disease and went on to develop empyema followed by chylothorax despite chemotherapy for fully sensitive M. tuberculosis. The pathogenesis of chylothorax remains speculative. The thoracic duct and its collaterals could be obstructed by either the paravertebral mass or by the diffuse lymphatic infiltration. It is equally possible that the thoracic duct could have been eroded by either the granulomatous process or by the intrapleural streptokinase itself. While tuberculosis itself is a well-recognised cause of chylothorax, the temporal relation to the use of streptokinase in our case is significant and may be aetiologically related. As such, further data is required on all the possible complications of intrapleural streptokinase as well as to ascertain the optimal regimen for such therapy.
We suggest that for patients with tuberculous abscess that is not surgically debrided, consideration should be given to increasing the dosage and duration of antituberculous chemotherapy. AFB organisms in these patients may be viable for many weeks after commencing treatment and an attempt should be made to reculture further specimens when available. The pleural extension of the abscess in our patient simplified specimen taking.
Clinicians should be aware of the possibility of damage to the thoracic duct as a result either of tuberculous abscess extension or of intrapleural instillation of streptokinase.
References
Hayword AC, Watson JM . Tuberculosis in England and Wales 1982–93; notifications exceeded predictions. Comm Dis Rep. 1995; 5: R29–R33.
Task force of the European Respiratory Society, World Health Organisation and the Europe region of the International Union against Tuberculosis and Lung disease. Tuberculosis management in Europe. Eur Respir J 1999; 14: 978–992.
Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax 1998; 53: 536–548.
Moon MS . Tuberculosis of the spine – controversies and a new challenge. Spine 1997; 22: 1791–1797.
Anonymous. Case no. 1. Pott's disease with a large psoas abscess. Tech Urol 1998; 4: 99–100.
Strange C, Sahn SA . Management of parapneumonic pleural effusions and empyema. In: Wallace RJ (ed). Infections Disease Clinics of North America. Saunders: Philadelphia, 1991, pp 539–559.
Tillet WS, Sherry S . The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal des-oxyribonuclease on fibrinous, purulent and sanguinous pleural exudations. J Clin Invest 1949; 28: 173–190.
Godley PJ, Bell RC . Major haemorrhage following administration of intrapleural streptokinase. Chest 1984; 86: 486–487.
Aye RW, Froese DP, Hill LD . Use of purified streptokinase in empyema and haemothorax. Am J Surg 1991; 161: 560–562.
Davies RJO, Traill ZC, Gleeson FV . Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997; 52: 416–421.
Barletta JF . Streptokinase and urokinase for the treatment of pleural effusions and empyema [Review]. Ann Pharm 1999; 33: 495–498.
Yater WM . Nontraumatic chylothorax and chylopericardium: review and report of a case due to carcinomatous thomboangitis obliterans of the duct and upper great veins. Ann Intern Med 1935; 9: 600–616.
Menzies R, Hidvegi R . Chylothorax associated with tuberculous spondylitis. Can Assoc Radiol J 1988; 39: 238–241.
Author information
Authors and Affiliations
Additional information
Funding was from departmental sources
Rights and permissions
About this article
Cite this article
Prasad, R., Fraser, M., Urquhart, G. et al. Rupture of tuberculous spinal abscess resulting in tuberculous empyema and chylothorax. Spinal Cord 41, 410–412 (2003). https://doi.org/10.1038/sj.sc.3101449
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101449