Abstract
Study design: Case report.
Objective: To report three cases of spinal intramedullary lipoma seen in the last 10 years and present the clinical characteristics and surgical outcome of these cases.
Method: Two patients were boys aged 12 years and 7 months, respectively. The other was a female patient aged 6 months. Chief complaints were hemiparesis, back swelling and thoracic scoliosis. All patients were diagnosed with magnetic resonance images. The lesion was located in the cervico-thoracic spine (foramen magnum to T1) in one case, thoracic spine (T9−T12) with the back swelling at L2-4 level in the second, and in the third, one mass extended from C6 to T11 and the other mass was located in the L1-2 level, separately.
Result: All masses were removed subtotally and dysraphism was absent. Postoperatively, neurological status of the first and the second patient were unchanged, but in the third case weakness was transiently aggravated.
Conclusion: Intramedullary lipoma is a rare spinal lesion and multiple intramedullary lipoma is extremely rare. Treatment principle is surgical decompression before symptom progression. Laminoplastic laminotomy is an appropriate approach for decompression of an intramedullary lipoma.
Similar content being viewed by others
Introduction
Spinal intramedullary lipoma is a rare lesion and accounts for 1% of all spinal masses. The usual location is in the cervico-thoracic region, mostly extradural with an association with spinal dysraphism.1,2,3,4,5,6 A sacral or lumbosacral defect that communicates with a subcutaneous lipoma is a common finding.1,2,3 Lipomas unassociated with spinal dysraphism are rare and few cases have been reported.3,4,5,7 We experienced three cases of spinal intramedullary lipoma and the lesions were located in the cervical, thoracic, and cervico-thoracic regions. There was no spinal dysraphism. The third case in the present series was holocord and multiple lipoma, which is an extremely rare spinal lesion.5,7 We present these cases with the clinical characteristics, surgical management and clinical outcome.
Case report
Case 1
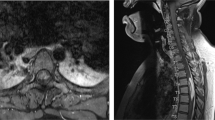
A 6-month-old girl presented with a left hemiparesis. At the age of 2 months, left hemiparesis developed and progressed. Neurological examination on admission showed a left hemiparesis (ASIA impairment scale, left upper extremity: C, left lower extremity: D) and ankle clonus on both sides. Skin stigmata such as hair, dimple, or mass were not found. Spine MRI revealed intramedullary lipoma extending from the foramen magnum to T1 (Figures 1a, b). This mass occupied nearly the full diameter of the spinal cord. A midline suboccipical craniectomy and total laminectomy of C1 with laminoplastic laminotomy from C2 to C7 was performed. A laminoplastic laminotomy was used for access to the intramedullary lipoma. After incision of the interspinous ligament and bilateral partial laminectomy of the lowest desired level, the ligamentum flavum was incised. Then longitudinal cutting of lamina with an electrical saw was performed from the lowest desired lamina to the uppermost desired lamina. Using the interspinous ligament of the uppermost lamina as a hinge, the spinous process, interspinous process, lamina and ligamentum flavum were elevated from the dura. After removal of the mass, these were repositioned and holes were made on the residual lamina, and then were fixed with silk ties. As the object of laminoplastic laminotomy in these patients was not an expansion of the canal but surgical access route for mass removal, we repositioned the posterior element without expansion.
(a) T1-weighted sagittal image shows a huge homogeneous high signal lesion from foramen magnum to T1 (arrow). On the high cervical level, the brain stem is deviated anteriorly. (b) T1-weighted axial image shows that lipoma compromises full diameter of the cord. The cord is compressed anteriorly and to the right (white arrow) and thin. (c) At 2 years after operation, spinal alignment is well preserved
There was no spinal dysraphism. The intramedullary lipoma was intermingled with left-sided nerve roots and the margin was poor. The lipoma was removed subtotally and nerve roots intermingled with the fat were saved. Postoperatively, neurological status was nearly the same as preoperative status. At 2 years after operation, cervical-spine radiography showed no progression of kyphosis (Figure 1c). With physiotherapy, the left side motor power improved (ASIA impairment scale, left upper extremity; D, left lower extremity; D), 8 years after surgery.
Case 2
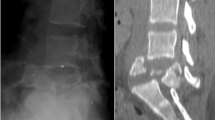
A 7-month-old boy presented with a low back mass. The mass was detected at birth and was covered with a thin membrane, which became thicker with time. There were no neurological or urological problems. Spine MRI showed an intradural mass at the dorsal surface of the spinal cord from T9 to T12. The skin mass, which was located from L2 to L4, was of the same signal intensity as the intradural lesion (Figure 2). Although there was no neurological deficit, we removed the masses to prevent deterioration and to promote cosmetic effect. Laminoplastic laminotomy (from T8 to T12, the method was same as case 1) was performed and no dysraphism was found at that level. The mass was located in the subpial space. As the mass was intermingled with spinal cord, the mass was removed subtotally. The back mass was removed after an additional skin incision and there was no connection between the two masses. This mass showed the same gross finding as the spinal one. Pathological diagnosis was lipoma. After operation, no neurologic deficit was found.
(a) T1-weighted sagittal image shows a dorsally located mixed high and low signal lesion (black arrow). The lesion is located from T9 to T12 and compresses the cord anteriorly. There is no tethering. Skin lesion is located from L2 to L4 and has iso-signal intensity same as spinal lesion without connection with the intradural mass (white arrow). (b) T1-weighted axial image reveals that the lesion is high signal lesion and dorsally located. The spinal cord is compressed and thin (white arrow)
Case 3
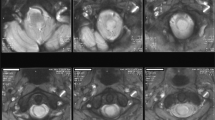
A 12-year-old boy presented with scoliosis. During the work-up for the scoliosis, a spinal intramedullary mass was detected. When he was 1 year old, he underwent craniotomy due to a right temporal astrocytoma. On admission, he showed bifrontal alopecia, bitemporal erythematous skin lesions and erythematous elevated skin lesions on the forehead and lip. There was no stigma on the lumbar skin. He was noted to have a left hemiparesis (ASIA impairment scale, left upper extremity; D, left lower extremity; D) and long tract signs. Spine MRI showed intramedullary spinal mass from C6 to T11 and another isolated mass between L1 and L2. These masses showed the same signal intensities on the MRI. However, there was no connection (Figure 3a, b). As there were spinal lesions and right brain atrophy, it was difficult to determine the origin of his weakness. Both median nerve and posterior tibial nerve somato-sensory-evoked potentials revealed a central conduction defect, and there was no radiculopathy or peripheral nerve problem on electromyography and nerve conduction study. However, with a normal right internal capsule and left-sided central conduction defect, which was documented by somato-sensory-evoked potential, the weakness was thought to originate from the spinal lesion rather than by brain atrophy. As the scoliosis was of a significant degree and potentially progressive, lipoma debulking before scoliosis correction was needed. Laminoplastic laminotomy (from C5 to L3, the method was same as case 1) was performed and there was no spinal dysraphism or tethering. The lumbar spinal mass was subtotally removed first and then the upper lesion was removed subtotally. Distinction between the mass and spinal cord was poor and the mass was intermingled with the nerve roots. Pathological examination showed uniformly mature adipose tissue, intersected by connective tissue and vessels, which suggested lipoma. The fibrous tissue is composed of immature mesenchymal cells (Figure 3c). Postoperatively, the low extremity weakness was aggravated transiently (ASIA impairment scale, left upper extremity; D, left lower extremity; C). There was no change of urological function. After 4 months, his left side motor power improved to the preoperative state.
(a) T1-weighted sagittal image shows high signal intensity intradural lesion from C6 to T11 and another isolated mass on the L1–L2. These masses have same signal intensity and have no connection. (b) T1-weighted axial image reveals that the lesion has high signal intensity and compresses the cord to the right and anterior direction (arrow). This mass occupies 70% of the canal area. (c) Lipoma consisted of mature adipocytes has only a slight variation in cellular size and shape. It is intermixed with adjacent immature mesenchymal tissue (H&E, ×100)
Discussion
Several hypotheses are proposed as the origin of the intramedullary lipoma. The first is ‘developmental error theory’. Inclusion of the misplaced adipocytes during the formation of the neural tube causes growth of lipoma in the spinal cord.1,3,4,6,7,8,9,10. This is not a true neoplasm but a hamatoma or a malformation.8 This theory also explains the dorsal location of lipoma and may explain spinal lipoma without dysraphism.1,3,4,6,7,8,11. Growth of lipoma displaces the normal spinal cord laterally and thus lipoma was located between dorsal roots.7 The second hypothesis is ‘metaplasia theory’. Connective tissue metaplasia may lead to deposition of fat within the dura.8,9 The third is ‘hamartomatous origin theory’. The fat tissue can include peripheral nerve twig, dermoid cyst, skeletal muscles and lymphoid or renal tissue, which originate from ectoderm or mesoderm.5,8,11 The fourth hypothesis is that adipocytes could arise from cells giving rise to the spinal vessels. In normal conditions, mesenchymal cells form the spinal vessel and these cells are prevented from forming adipocyte by neural crest cells. However, if neural crest cells are defective, the inhibition fails and mesenchymal cells forms adipocytes.12 All of these theories share some basic aspects but none of these theories fully explain the exact genesis of the spinal lipoma. However, the first hypothesis is commonly accepted.
A slow ascending spastic monoparesis or paraparesis is a common initial symptom in cases of lipoma affecting the cervical or thoracic region.2,5 Their dorsal location causes ventral flattening of the cord.6 The clinical course of intramedullary spinal lipoma is characteristic. Initial symptom progression is slow and late deterioration is rapid.2,3,4,5 Slow enlargement of the lipoma allows for accommodation of the cord without functional change. A point may be eventually reached when there is no longer any physiologic reserve, and neurological dysfunction then rapidly progresses.3 Usually, postoperative neurological status is not better than the preoperative one.3 Lee et al,3 explained as follows: intraspinal lipomas are probably congenital lesions, and therefore, they would not only compress normal tissue, but would have actually replaced normal tissue during development. There would be less redundancy in the functional pathways, and, therefore, a greater chance of permanent injury to the function of the normal spinal cord. If an initial mass is large, then symptoms present in early childhood.3,10 Spinal lipoma without dysraphism has only a small space for expansion and thus an early presentation of symptoms.5 The first case showed only a 2-month lag time before symptoms.
Spinal lipoma is a congenital lesion and not a neoplasm, and histologically, spinal intramedullary lipoma is an admixture of lobulated fatty tissue separated by delicate connective tissue and intervening neural tissues.1,5 Therefore, if they are asymptomatic they may be left alone without treatment.1 However, if symptoms progress, surgical debulking is recommended. Aggressive removal of this mass is not necessary. Even if patients are symptomatic, decompression only is sufficient.1,2 Furthermore, the infiltrative nature of the lipoma hinders gross total removal.1,2 Early surgical decompression prevents irreversible spinal cord dysfunction, because most symptomatic patients usually do not improve after surgery.3,6,7,9 Therefore, the operative principle is decompression before symptom progression.
As most patients are young and lipoma is a benign lesion, spinal stability and cosmetic results after operation are important. Therefore, laminoplastic laminotomy is an appropriate approach for decompression of intramedullary lipoma.
References
Dyck P . Intramedullary lipoma. Diagnosis and treatment. Spine 1992; 17: 979–981.
Kodama T, Numaguchi Y, Gellad FE, Sadato N . Magnetic resonance imaging of a high cervical intradural lipoma. Comput Med Imag Graph 1991; 15: 93–95.
Lee M et al. Intramedullary spinal cord lipomas. J Neurosurg 1995; 82: 394–400.
Kujas M, Sichez JP, Lalam TF, Poirier J . Intradural spinal lipoma of the conus medullaris without spinal dysraphism. Clin Neuropathol 2000; 19: 30–33.
Timmer FA, van Rooij WJ, Beute GN, Teepen JL . Intramedullary lipoma. Neuroradiology 1996; 38: 159–160.
Razack N, Jimenez OF, Aldana P, Ragheb J . Intramedullary holocord lipoma in an athlete: case report. Neurosurgery 1998; 42: 394–396.
Patwardhan V, Patanakar T, Armao D, Mukherji SK . MR imaging findings of intramedullary lipomas. Am J Roentgenol 2000; 174: 1792–1793.
Ammerman BJ, Henry JM, De Girolami U, Earle KM . Intradural lipomas of the spinal cord. A clinicopathological correlation. J Neurosurg 1976; 44: 331–336.
Matsui H, Kanamori M, Miaki K . Expansive laminoplasty for lumbar intradural lipoma. Int Orthop 1997; 21: 185–187.
Muraszko K, Youkilis A . Intramedullary spinal tumors of disordered embryogenesis. J Neurooncol 2000; 47: 271–281.
Li YC et al. Pathogenesis of lumbosacral lipoma: a test of the premature dysjunction theory. Pediatr Neurosurg 2001; 34: 124–130.
Catala M . Embryogenesis. Why do we need a new explanation for the emergence of spina bifida with lipoma? Child Nerv Syst 1997; 13: 336–340.
Acknowledgements
This work was partly supported by Korea Institute of Science and Technology Evaluation and Planning (KISTEP) and BK 21 Human Life Science, Republic of Korea.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, C., Wang, KC., Kim, SK. et al. Spinal intramedullary lipoma: report of three cases. Spinal Cord 41, 310–315 (2003). https://doi.org/10.1038/sj.sc.3101441
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101441
Keywords
This article is cited by
-
Nondystrophic holocord intramedullary lipoma: an uncommon case
Child's Nervous System (2018)
-
Non-dysraphic intradural spinal cord lipoma: case series, literature review and guidelines for management
Acta Neurochirurgica (2010)
-
Intramedullary lesions of the conus medullaris: differential diagnosis and surgical management
Neurosurgical Review (2009)