Abstract
Study design: A case report.
Objectives: To report a case of cervical amyotrophy caused by hypertrophy of the posterior longitudinal ligament (HPLL).
Setting: Department of Neurological Surgery, Aichi Medical University, Aichi, Japan.
Methods: The patient had severe muscular atrophy in the deltoid and triceps with slight localized hypesthesia in the C5 area and severely unstable gait due to diminished vibration sense in the knees and ankles. Magnetic resonance imaging (MRI) showed expanded cord compression from C4 to C6 with intramedullary high-signal intensity due to HPLL. Transverse image MRI was useful to identify the HPLL.
Results: Resection of HPLL was achieved by an anterior approach. Histological findings of the surgical specimens showed thickening of the ligamentous tissue with proliferation of chondrocytes.
Conclusions: HPLL should be included as a causative pathology of cervical spondylotic amyotrophy. Careful neurological examination including sensory examination of the lower limbs should be performed to avoid confusion with motor neuron disease.
Similar content being viewed by others
Introduction
Muscular atrophy in the upper extremities with insignificant sensory loss is occasionally caused by cervical spondylosis,1,2 and this condition is called cervical spondylotic amyotrophy. One of the possible mechanisms causing this syndrome is multi-segmental damage to the anterior horns in a narrowed canal by dynamic motion.3 To our knowledge, hypertrophy of the posterior longitudinal ligament (HPLL), defined as pathological thickening of the ligamentous tissue,4,5,6,7,8,9,10 causing this particular syndrome has never been reported.
We describe a case of cervical amyotrophy caused by HPLL.
Case Report
A 56-year-old man was admitted to our hospital with a 1-year history of motor weakness of the left arm. Physical examination showed severe atrophy in the left shoulder girdle including the deltoid, supra- and infra-spinatus, suprascaplar muscles and the proximal portion of the left arm including the biceps and triceps muscles. Neurological examination showed moderate motor weakness of the left upper and lower limbs and clumsiness of fingers. The biceps reflex was diminished on both sides, and the left triceps reflex was slightly hyperactive. Deep tendon reflexes in the left knee and ankle joints were hyperactive and severely unstable gait was present. Sensory changes showed significant reduction of vibration sense in the knees and ankles, predominantly on the left, and slight hypesthesia only in the C5 region. Electromyographic findings were compatible with denervation of the muscles innervated by C5, C6 and C7 bilaterally with left-side predominance.
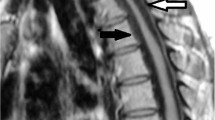
A cervical radiograph showed a slight spondylotic change without ossification of the posterior longitudinal ligament (OPLL) at C4/5 and C5/6 (Figure 1). Sagittal T2-weighted magnetic resonance imaging (MRI) showed multi-segmental cord compression from C4 to C6 with intramedullary high-signal intensity lesions. On transverse T2-weighted MRI, the spinal cord showed an anteroposterior deformity due to the epidural compression at the midvertebral body level as well as at the vertebral endplate level from C4 to C6. A herniated intervertebral disc (HID) was visualized as a central low-signal intensity mass, and HPLL as an iso-signal intensity mass bilaterally in the ventral spinal canal (Figure 2). Based on these clinical and radiographic aspects, a diagnosis of cervical amyotrophic myelopathy caused by HPLL associated with HID was made. Surgery was planned to decompress the spinal cord by removing the hypertrophic ligament from C4 to C6 as well as the HID at C4/5 and C5/6.
T2-weighted MRI. (a) Sagittal image showing cord compression at the midvertebral body level as well as at the vertebral endplate level from C4 to C6. (b–e) Transverse images showing an iso-signal intense HPLL (wide arrow) as well as a low to iso-signal intense HID (small arrow) and osteophyte (large arrow) compressing the spinal cord with a high-signal intensity in the anterior horns with left-side predominance (asterisk)
A microsurgical anterior approach was performed. After a 1.8 cm wide partial vertebrectomy at C4/5 and C5/6 using a microsurgical saw, the hypertrophic ligament and HID were removed en bloc. The dura was intact. After satisfactory decompression at both levels, the dura became full and pulsatile. Autogenous vertebral grafts with ceramic plates in a sandwich fashion were carried out at C4/5 and C5/6 (Figure 3).
The surgical specimens showed thickening of the ligament at the level of C5, with the diameter ranging from 3 to 4 mm. On hematoxylin-eosin (HE) stainings, there was proliferation of chondrocytes with calcification and fibrous tissue adjacent to the small vessels accounting for HPLL. There was no significant ossification in the ligamentous tissue (Figure 4).
(a) Photomicrograph of surgical specimen showing thickened ligament with associated HID from C4 to C6 (HE stainings, original magnification, ×8). High-power photomicrographs of HPLL showing (b) Herniated intervertebral disc prolapsed into the deep layer of the PLL. Note proliferation of small cells with degenerated ligamentous fibers (arrow) and (c) proliferation of chondrocytes in the PLL (HE stainings, original magnification, ×200)
Postoperatively, weakness of the upper extremities and the clumsy hand improved gradually although the muscular atrophy remained unchanged. His neurological condition has been stable for 3 years.
Discussion
Syndromes with severe muscular atrophy and weakness of the upper extremities in patients with cervical spondylosis have been well documented.11,12 Due to absent or insignificant sensory deficits, this syndrome is sometimes confused with motor neuron disease (MND).3,8 In the current case as well, the prominent muscular atrophy in the upper limbs, clumsiness of fingers and localized slight hypesthesia in the C5 region were characteristic of MND. However, in addition to these neurological deficits, an unstable gait due to diminished vibration sense in the knees and toes was present. The posterior column involvement in this case indicated that pure MND could be neurologically ruled out. It is thus important to perform careful neurological examination including sensory examination of the lower limbs to avoid confusion with MND.
Whether the pathogenesis of this syndrome is selective damage to the ventral nerve roots or to the anterior horns of the spinal cord parenchyma is not clearly elucidated.6,7,12 In the present case, an intramedullary high-signal intensity lesion appeared in the central gray matter to the anterior horn of the compressed cord on transverse T2-weighted MRI. Such intramedullary high-signal intensity lesions are referred to as `snake-eyes appearance10 or fried-egg appearance13, and are thought to be irreversible necrosis, rather than reversible edema.10,14,15 Several authors have reported this pathological change in autopsy findings of cervical compression myelopathy.6,11,16 Therefore, the muscular atrophy of the present case might have been caused by multisegmental damage of the anterior horns due to HPLL, unlike the theory of damage of the ventral nerve roots proposed by Keegan.7 Wada et al.15 documented that single-segment injury of the anterior horn cells would not be sufficient to develop muscular atrophy. We thus consider HPLL from C4 to C6 to have been the major factor in the muscular atrophy of this patient.
Hypertrophy of the posterior longitudinal ligament was first described by Kamikozuru et al.17 in 1974. Since then, several authors have reported myelopathy induced by HPLL in the English literature.4,5,9,12,18,19,20 The histological characteristics of these cases include association of HID, absence or insignificant findings of OPLL and abnormal thickening of the posterior longitudinal ligament (PLL) greater than 3.5 mm in anteroposterior diameter. In the current case as well, HPLL was associated with HID without significant ossification. The thickened ligament was mainly composed of a proliferation of fibrocartilageous tissues with hyperplasia of chondrocytes both in the deep layer and in the superficial layer of the PLL. This change began at the margin of HID, where a proliferation of small vessels had formed. The normal structure of the ligamentous tissue was interrupted. On the basis of these histological findings, we hypothesize that metaplasia of fibroblasts of the collagenous tissues to chondrocytes resulting in formation of fibrocartilage was the mechanism of HPLL in our patient. HID prolapsing into the ligament and proliferation of small vessels may play an important role in initiating metaplasia.9 Ossification was observed, but was insufficient to make a diagnosis of classic OPLL. Sequential transverse T2-weighted MRI well visualized HPLL in addition to the associated HID. HPLL, apparent as isointensity, together with HID in the present case compressed the spinal cord at the midvertebral body level as well as the vertebral endplate level from C4 to C6. Several investigators have documented the usefulness of MRI in making a diagnosis of HPLL.21,22,23 Yamazaki et al.23 suggested that the normal portion of the PLL was not visualized, although the thickened portion was clearly shown, using spin echo sequences. Therefore, one must consider HPLL when cord compression is expanded to the midvertebral body beyond the disc spaces. Appropriate vertebrectomy with direct removal of HPLL with HID is mandatory for myelopathy induced by HPLL.
In conclusion, multi-segmental HPLL associated with HID should be differentiated as a causative pathology of cervical spondylotic amyotrophy. It is essential to perform careful neurological examination including sensory examination of the lower limbs to avoid a mis-diagnosis of MND.
References
Brain WR, Northfield D, Wilkinson M . The neurological manifestations of cervical spondylosis Brain 1952 75: 187–225
Crandall PH, Batzdorf U . Cervical spondylotic myelopathy J Neurosurg 1966 25: 57–66
Dorsen M, Ehni G . Cervical spondylotic radiculopathy producing motor manifestations mimicking primary muscular atrophy Neurosurgery 1979 5: 427–431
Epstein NE . Ossification of the posterior longitudinal ligament: Diagnosis and surgical treatment Neurosurg Q 1992 2: 223–241
Epstein NE . Advanced cervical spondylosis with ossification of the posterior longitudinal ligament and resultant neurological sequelae J Spinal Disord 1996 9: 477–484
Ito T et al. The clinical consideration of the dissociated motor loss syndrome (Keegan) in disease of the cervical spine Nippon Seikeigeka Gakkai Zasshi 1980 54: 135–151 in Japanese
Keegan JJ . The cause of dissociated motor loss in the upper extremity with cervical spondylosis J Neurosurg 1965 23: 528–536
Liversedge LA, Hutchinson EC, Lyons JB . Cervical spondylosis simulating motor-neuron disease Lancet 1953 2: 652–655
Mizuno J, Nakagawa H . Analysis of hypertrophy of the posterior longitudinal ligament of the cervical spine, on the basis of clinical and experimental studies Neurosurgery 2001 49: 1091–1098
Ramanauskas WL et al. MR imaging of compressive myelomalacia J Comput Assist Tomogr 1989 13: 399–404
Mizuno J et al. Pathological study of ossification of the posterior longitudinal ligament (OPLL) with special reference to mechanism of ossification and spinal cord damage Spinal Surgery 1988 2: 81–87 in Japanese
Yanagi T, Kato K, Sobue I . Cervical spondylotic amyotrophy simulating motor neuron disease Rinsho Shinkeigaku 1976 16: 520–528 in Japanese
Iwasaki Y et al. CT myelography with intramedullary enhancement in cervical spondylosis J Neurosurg 1985 63: 363–366
Kameyama T et al. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament Brain 1995 118: 263–278
Wada E, Okudera M, Yonenobu K . Intramedullary changes of the spinal cord in cervical spondylotic myelopathy Spine 1995 20: 2226–2232
Hashizume Y et al. Pathology of spinal cord lesions caused by ossification of the posterior longitudinal ligament Acta Neuropathol (Berl) 1984 63: 123–130
Kamikozuru M, Yamaura I . Hypertrophy of the cervical posterior longitudinal ligament (a provisional name) causing myelopathy A case report. Presented at the Second Annual Meeting of the Japan Spine Research Society, Tokyo, Japan, November 9, 1974 (in Japanese
Hase H et al. Severe cervical myelopathy of the cervical posterior longitudinal ligament: A case report Spine 1992 17: 1417–1421
Mizuno J et al. Compression myelopathy due to hypertrophy of the posterior longitudinal ligament with herniated intervertebral discs. Case report Neuro-Orthopedics 1992 13: 113–120
Motegi H et al. Proliferation cell nuclear antigen in hypertrophied spinal ligaments: immunohistological localization of proliferating cell nuclear antigen in hypertrophied posterior longitudinal ligament of the cervical spine Spine 1998 23: 305–310
Sakamoto R . Comparative study between MRI and histological findings in the area of ossification and calcification of the ligaments. Investigation Committee Report on the Ossification of the Spinal Ligaments of the Japanese Ministry of Public Health and Welfare in 1989 Edited by T. Kurokawa Tokyo, Japanese Ministry of Health and Welfare 1990 pp. 180–182 in Japanese
Yamaura I . Degeneration of intervertebral disc and change of the posterior longitudinal ligament: Microscopic findings of the specimen obtained in the biopsy Investigation Committee Report on the Ossification of the Spinal Ligaments of the Japanese Ministry of Public Health and Welfare in 1989 Edited by T. Kurokawa Tokyo, Japanese Ministry of Health and Welfare 1990 pp 180–182 in Japanese
Yamazaki A et al. Magnetic resonance imaging and histologic study of hypertrophic cervical posterior longitudinal ligament. A case report Spine 1992 16: 1262–1266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mizuno, J., Nakagawa, H. & Hashizume, Y. Cervical amyotrophy caused by hypertrophy of the posterior longitudinal ligament. Spinal Cord 40, 484–488 (2002). https://doi.org/10.1038/sj.sc.3101321
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101321
Keywords
This article is cited by
-
Cervical spondylotic amyotrophy: a systematic review
European Spine Journal (2019)