Abstract
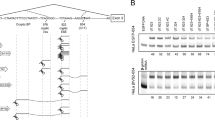
Most eukaryotic messenger RNAs have the sequence AAUAAA 11–30 nucleotides from the 3′-terminal poly(A) tract1,2. Since this is the only significant sequence homology in the 3′ non-coding region it has been suggested that it may be a recognition site for enzymes involved in polyadenylation and/or termination of polymerase II transcription2–4. This idea is strengthened by observations on the effect of deletion mutations in or around the AATAAA sequence on polyadenylation of late simian virus 40 (SV40) mRNA; removal of this sequence prevents poly(A) addition3. Naturally occurring variants ofthis hexanucleotide are rare5–11 and hitherto their functional significance has not been assessed. We have now identified a human α2-globin gene which contains a single point mutation in this hexanucleotide (AATAAA → AATAAG). The paired α1 gene on the same chromosome is completely inactivated by a frame-shift mutation. This unique combination has enabled the expression of the mutant α2 gene to be studied in vivo where it has been found that the accumulated level of α2-specific mRNA in erythroid cells is reduced. Furthermore, readthrough transcripts extending beyond the normal poly(A) addition site are detected in mRNA obtained from HeLa cells transfected with cloned DNA from the mutant α2 gene, suggesting that the single nucleotide change in the AATAAA sequence is the cause of its abnormal expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Proudfoot, N. J. & Brownlee, G. G. Nature 263, 211–214 (1976).
Proudfoot, N. J. Nature 298, 516–517 (1982).
Fitzgerald, M. & Shenk, T. Cell 24, 251–260 (1981).
Ziff, E. B. Nature 287, 491–499 (1980).
Hagenbuchle, O., Krikeles, M. S. & Sprague, K. U. J. biol. Chem. 254, 7157–7169 (1980).
Jung, A., Sippel, A. E., Grez, M. & Schutz, G. Proc. natn. Acad. Sci. U.S.A. 77, 5759–5763 (1980).
Fornwald, J., Kuncio, G., Peng, I. & Ordahl, C. P. Nucleic Acids Res. 10, 3861–3876 (1982).
Zakut, R. et al. Nature 298, 857–859 (1982).
Capon, D. J., Chen, E. Y., Levinson, A. D., Seeburg, P. H. & Goeddel, D. V. Nature 302, 33–37 (1983).
Donehower, L., Huang, A. & Hager, G. J. Virol. 37, 226–238 (1981).
Setzer, D. R., McGrogan, M., Nunberg, J. H. & Schimke, R. T. Cell 22, 361–370 (1980).
Pressley, L. et al. New Engl. J. Med. 303, 1383–1388 (1980).
Higgs, D. R. et al. Proc. Natn. Acad. Sci. U.S.A. 78, 5833–5837 (1981).
Maniatis, T., Fritsch, E. F. & Sambrook, J. in Molecular Cloning, a Laboratory Manual (Cold Spring Harbor Laboratory, New York, 1982).
Rimm, D. L., Horness, D., Kucera, J. & Blattner, F. R. Gene 12, 301–308 (1980).
Orkin, S. H. & Goff, S. C. Cell 24, 345–351 (1981).
Liebhaber, S. A. & Kan, Y. W. J. clin. Invest. 68, 439–446 (1981).
Chang, J. C., Kan, Y. W., Trecartin, R. F. & Temple, G. F. Proc. natn. Acad. Sci. U.S.A. 76, 2886–2889 (1979).
Trecartin, R. F. et al. J. Clin. Invest. 68, 1012–1017 (1981).
Kinniburgh, A. J. et al. Nucleic Acids Res. 10, 5421–5427 (1982).
Banerji, J., Rusconk, S. & Schaffner, W. Cell 27, 299–308 (1981).
Busslinger, M., Moschonas, N. & Flavell, R. A. Cell 27, 289–298 (1981).
Grosveld, G. C., De Boer, E., Shewmaker, C. K. & Flavell, R. A. Nature 295, 120–126 (1982).
Treisman, R., Proudfoot, N. J., Shander, M. & Maniatis, T. Cell 29, 903–911 (1982).
Nevins, J. R. & Darnell, J. E. Cell 15, 1477–1493 (1978).
Fraser, N. W., Nevins, J. R., Ziff, E. & Darnell, J. E. J. molec. Biol. 129, 643–656 (1979).
Nevins, J. R., Blanchard, J.-M. & Darnell, J. E. J. molec. Biol. 144, 377–386 (1980).
Ford, J. P. & Hsu, M. T. J. Virol. 28, 795–801 (1978).
Hofer, E. & Darnell, J. E. Jr Cell 23, 585–593 (1981).
Darnell, J. E. Nature 297, 365–371 (1982).
Michelson, A. M. & Orkin, S. H. Cell 22, 371–377 (1980).
Liebhaber, S. A., Goossens, M. J. & Kan, Y. W. Proc. natn. Acad. Sci. U.S.A. 77, 7054–7058 (1980).
Orkin, S. H., Goff, S. C. & Hechtman, R.L. Proc. natn. Acad. Sci. U.S.A. 78, 5041–5045 (1981).
Michelson, A. & Orkin, S. J. biol. Chem.(in the press).
Maxam, A. M. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Hunt, D. M. et al. Br. J. Haemat. 45, 53–64 (1980).
Favaloro, J. M., Treisman, R. H. & Kamen, R. Meth. Enzym. 65, 718–749 (1980).
Alwine, J. C., Kemp, D. J. & Stark, G. R. Proc. natn. Acad. Sci. U.S.A. 74, 5350–5354 (1977).
Lauer, J., Shen, C-K. J. & Maniatis, T. Cell 20, 119–130 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higgs, D., Goodbourn, S., Lamb, J. et al. α-Thalassaemia caused by a polyadenylation signal mutation. Nature 306, 398–400 (1983). https://doi.org/10.1038/306398a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/306398a0
This article is cited by
-
Deep learning of human polyadenylation sites at nucleotide resolution reveals molecular determinants of site usage and relevance in disease
Nature Communications (2023)
-
Context-specific regulation and function of mRNA alternative polyadenylation
Nature Reviews Molecular Cell Biology (2022)
-
An atlas of alternative polyadenylation quantitative trait loci contributing to complex trait and disease heritability
Nature Genetics (2021)
-
Genetic epidemiology of hemoglobinopathies among Iraqi Kurds
Journal of Community Genetics (2021)
-
Alpha thalassemia genotypes in Kuwait
BMC Medical Genetics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.