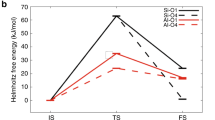
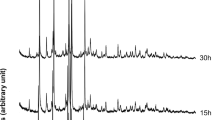
Abstract
The crystal structure of a high silica zeolite ZSM-39, unit cell composition excluding residual water and occluded materials—(Na, tetramethylammonium ion, tetraethylammonium ion)0.4 (AlO2)0.4 (SiO2)135.6, was determined by X-ray powder diffraction. The framework is pseudoface-centred, pseudocubic with a = 19.36±0.02 Å and ideal symmetry Fd3m. The framework consists of a space-filling arrangement of pentagonal dodecahedra and hexakaidecahedra and is isostructural with the 17 Å cubic gas hydrate. The ZSM-39 framework is composed entirely of five- and six-rings which limits its sorptive and exchange properties. However, the large fraction of five-rings and the high Si/Al ratio (>40) impart a high thermal stability. ZSM-39 containing no aluminium constitutes the end member composition. Although ZSM-39 is the only synthetic zeolite analogue of a gas hydrate, we propose here two related hypothetical frameworks containing pentagonal dodecahedral cages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kamb, B. Science 148, 232 (1965).
McMullon, R. K., Mak, T. C. W. & Jeffrey, G. A. J. chem. Phys. 44, 2338 (1966).
Feil, D. & Jeffrey, G. A. J. chem. Phys. 35, 1863 (1961).
von Lasaux, A. Neues Jb. Miner. Abh. 250, (1876).
Skinner, B. J. & Appleman, D. E. Am. Miner. 218, 854 (1963).
Appleman, D. E., Amer. Crystallogr. Ass., Mineral. Soc. Amer. Jt Meet., Gatlinburg, 80 (1965).
Pauling, L. & Marsh, R. E., Proc. natn. Acad. Sci. U.S.A. 38, 112 (1952).
Claussen, W. F. J. chem. Phys. 19, 1425 (1951).
Claussen, W. F. J. chem. Phys. 19, 112 (1951).
Gros, G., Ponchard, M., Hagenmueller, P. J. Solid-State Chem. 2, 570 (1970).
Dwyer, F. G. & Jenkins, E. E. U.S. Patent 4,287,166 (1981).
Baerlocher, Ch., Hepp, A. & Meier, W. M. DLS-76, A Program for the Simulation of Crystal Structures by Geometric Refinement (Zurich, 1977).
Smith, D. K. A Revised Program for Calculating X-ray Powder Diffraction Patterns UCRL-502.64 (Lawrence Radiation Laboratories, 1967).
Žák, L. Am. Miner. 57, 779 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlenker, J., Dwyer, F., Jenkins, E. et al. Crystal structure of a synthetic high silica zeolite—ZSM-39. Nature 294, 340–342 (1981). https://doi.org/10.1038/294340a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/294340a0
This article is cited by
-
Structure-Property-Comparisons of Clathrasils and Gas Hydrates
Transactions of the Indian Institute of Metals (2019)
-
New silica clathrate minerals that are isostructural with natural gas hydrates
Nature Communications (2011)
-
Hydrothermal conversion of FAU zeolite into aluminous MTN zeolite
Journal of Porous Materials (2009)
-
Studies on the mechanism of ZSM-5 formation
Catalysis Letters (1991)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.