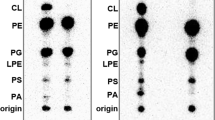
Abstract
Most secreted proteins of both eukaryotes and prokaryotes are synthesized as preproteins with NH2-terminal hydrophobic signal sequences which are removed during transfer of the proteins across the membrane1,2. The role of cleavable signal sequences in the insertion of proteins into the cytoplasmic membrane is not clear. Rothman and Lenard have proposed that the assembly of those integral membrane proteins that have a substantial extracytoplasmic domain (ectoproteins) occurs by a similar mechanism to that of secreted proteins, involving a cleavable signal sequence, but with complete secretion being prevented by a hydrophobic COOH–terminal region3. In eukaryotes, several ectoproteins seem to be synthesized in this way4–6 but in prokaryotes, the coat protein of M13 phage is the only reported example of a cytoplasmic membrane protein that is made as a preprotein2,7, although a non-cleavable signal-like sequence has been shown for the E. coli lactose permease8. We show here that penicillin-binding proteins 5 and 6 of Escheri-chia coli are the first examples of cytoplasmic membrane proteins, encoded by bacteria, that are processed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blobel, G. & Dobberstein, B. J. Cell Biol. 67, 835–851 (1975).
Wickner, W. Science 210, 861–868 (1980).
Rothman, J. E. & Lenard, J. Science 195, 743–753 (1977).
Rothman, J. E. & Lodish, H. F. Nature 269, 775–780 (1977).
Boothroyd, J. C., Cross, G. A. M., Hoeijmakers, J. H. J. & Borst, P. Nature 288, 624–626 (1980).
Singer, P. A., Singer, H. H. & Williamson, A. R. Nature 285, 294–300 (1980).
Chang, C. N., Model, P. & Blobel, G. Proc. natn. Acad. Sci. U.S.A. 76, 1251–1255 (1979).
Ehring, R., Beyreuther, K., Wright, J. K. & Overath, P. Nature 283, 537–540 (1980).
Spratt, B. G. Phil. Trans. R. Soc. B289, 273–283 (1980).
Spratt, B. G. Eur. J. Biochem. 72, 341–352 (1977).
Koyasu, S., Fukuda, A. & Okada, Y. J. Biochem. 87, 363–366 (1980).
Sutcliffe, J. G. Proc. natn. Acad. Sci. U.S.A. 75, 3737–3741 (1978).
Spratt, B. G., Boyd, A. & Stoker, N. G. J. Bact. 143, 569–581 (1980).
De Pedro, M. A. & Schwarz, U. Proc. natn. Acad. Sci. U.S.A. (in the press).
Waxman, D. J. & Strominger, J. L. J. biol. Chem. 256, 2059–2066 (1981).
Waxman, D. J. & Strominger, J. L. J. biol. Chem. 255, 3964–3976 (1980).
Waxman, D. J., Yocum, R. R. & Strominger, J. L. Phil. Trans. R. Soc. B289, 257–271 (1980).
Tipper, D. J. & Strominger, J. L. Proc. natn. Acad. Sci. U.S.A. 54, 1133–1141 (1965).
Zubay, G. A. Rev. Genet. 7, 267–287 (1973).
Cleveland, D. W., Fisher, S. G., Kirschner, M. W. & Laemmli, U. K. J. biol. Chem. 252, 1102–1106 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pratt, J., Holland, I. & Spratt, B. Precursor forms of penicillin-binding proteins 5 and 6 of E. coli cytoplasmic membrane. Nature 293, 307–309 (1981). https://doi.org/10.1038/293307a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/293307a0
This article is cited by
-
On the process of cellular division in Escherichia coli: Nucleotide sequence of the gene for penicillin-binding protein 3
Molecular and General Genetics MGG (1983)
-
Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium
Nature (1982)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.