Abstract
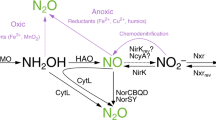
LABORATORY studies1–8 have indicated that chemical de-nitrification processes in nitrogen rich soils and nitrite solutions can produce NO or NO2 and perhaps methyl nitrite9. It has been suggested1–6 that self decomposition of nitrite produces various gaseous nitrogen compounds including nitric oxide (NO) and it was thought that NO was unlikely to escape from the soil because in aerobic soils NO is readily oxidised by molecular oxygen to NO2 and this in turn is rapidly adsorbed by soil materials and by soil water1,2. However the half life of oxidation is dependent on the NO concentration because it is a termolecular reaction10,11. At concentrations of 100 p.p.m. or greater (as in some studies) the half life for oxidation of NO to NO2 by atmospheric oxygen is one hour or less respectively, whereas at low concentrations (∼0.01 p.p.m.) the half life for this oxidation is of the order of 104 h. This variation in the oxidation rate by molecular oxygen explains why NO at low concentrations in the field can pass unoxidised from the soil to the atmosphere and perhaps explains why in some of the laboratory studies (at high concentrations) NO2 and not NO is detected. This NO exhalation in the field could not be detected previously due to the lack of a sufficiently sensitive measurement technique. We report here the first measurements indicating that NO is continuously exhaled from soils including ungrazed, unfertilised grassland. The measured rates of exhalation from the soil to the atmosphere are 0.06 to 0.73 × 10−11 kg nitrogen m−2 s−1 (equivalent to 0.2 to 2.3 kg N ha−1 yr−1). We show that this exhalation of NO is a significant source of nitrogen oxides (NOx) in the lower atmosphere as has been previously suggested12,13. These nitrogen oxides exert a direct influence on the ambient ozone and hydroxyl radical concentrations14 which in turn predominantly determine the oxidation rate of almost all substances in the troposphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Broadbent, F. E. & Clark, F. Soil Nitrogen Ch. 9, 344–359 (eds Bartholomew, W. V. & Clark, F. E.) Agronomy Monogr. No. 10 (Am. Soc. Agronomy, Madison, 1965).
Allison, F. E. Soil Nitrogen Ch. 16, 573–606 (eds Bartholomew, W. V. & Clark, F. E.) Agronomy Monogr. No. 10 (Am. Soc. Agronomy, Madison, 1965).
Nelson, D. W. & Bremner, J. M. Soil Biol. Biochem. 2, 203 (1970).
Bulla, L. A., Gilmour, C. M. & Bollen, W. B. Nature 225, 664 (1970).
Nömmik, N. & Thorin, J. Agrochimica 16, 319 (1972).
Van Cleemput, O., Patrick, W. H. & McIlhenny, R. C. J. Soil Sci. Soc. Am. 40, 55 (1976).
Moraghan, J. T. & Buresh, R. J. J. Soil Sci. Soc. Am. 41, 47 (1977).
Steen, W. C. & Stojanovic, B. J. Proc. Soil Sci. Soc. Am. 35, 277 (1971).
Stevenson, F. J. & Swaby, R. J. Proc. Soil Sci. Soc. Am. 28, 773 (1964).
Leighton, P. A. Photochemistry of Air Pollution (Academic, New York, 1961).
Demerjian, K. L., Kerr, J. A. & Calvert, J. G. Adv. environ. Sci. Technol. 4, 1–262 (1974).
Junge, C. E. Int. Geophys. Ser. 4, Air Chemistry and Radioactivity (Academic, New York, 1963).
Galbally, I. E. Tellus 27, 67 (1975).
Fishman, J. & Crutzen, P. J. J. geophys. Res. 82, 5897 (1977).
Regener, V. H. & Aldaz, L. J. geophys. Res. 74, 6935 (1969).
Galbally, I. E. Air Pollution Measurement Techniques. Special Environmental Report No.10, 179 (World Meteorological Organisation, Geneva, 1977).
Winer, A. M., Peters, J. W., Smith, J. P. & Pitts, J. N. Environ. Sci. Technol. 8, 1118 (1974).
Fontijn, A., Sabadell, A. J. & Ronco, R. J. Analyt. Chem. 42, 575 (1970).
Stevens, R. K. & Hodgeson, J. A. Analyt. Chem. 45, 443A (1973).
McIlroy, I. C. & Angus, D. E. CSIRO Div. Meteorol. Phys. Tech. Pap. No. 14 (1963).
Swinbank, W. C. CSIRO Div. Meteorol. Phys. Tech. Pap. No. 2 (1955).
CSIRO Riverina Laboratory, Div. Plant Indust. Annual Report (1968).
Northcote, K. H. Atlas of Australian Soils (CSIRO, Melbourne, 1962).
Kim, C. M. Soil Biol. Biochem. 5, 163 (1973).
Fed. Regist. 38, 15175 (1973).
Bundy, L. G. & Bremner, J. M. Commun. Soil Sci. Plant Analyt. 4, 179 (1973).
Söderlund, R. & Svensson, B. H. The Global Nitrogen Cycle (eds Svensson, B. H. & Söderlund, R.)in Nitrogen, Phosphorus and Sulphur—Global Cycles (SCOPE Report 7 Ecol. Bull., Stockholm, 1976).
Parker, B. C., Zeller, E. J., Heiskell, L. E. & Thompson, W. J. Antarctic J. U.S.A. 12, 133 (1977).
Parker, B. C., Heiskell, L. E., Thompson, W. J. & Zeller, E. J. Nature 271, 651 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GALBALLY, I., ROY, C. Loss of fixed nitrogen from soils by nitric oxide exhalation. Nature 275, 734–735 (1978). https://doi.org/10.1038/275734a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/275734a0
This article is cited by
-
Fixation of atmospheric nitrogen in the water by heat or light with the formation of nitrogen oxides
Doklady Biochemistry and Biophysics (2005)
-
Rates and controls of nitrous oxide and nitric oxide emissions following conversion of forest to pasture in Rondônia
Nutrient Cycling in Agroecosystems (2005)
-
Contemporary and pre-industrial global reactive nitrogen budgets
Biogeochemistry (1999)
-
Exchange of NO and NO2 between wheat canopy monoliths and the atmosphere
Plant and Soil (1996)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.