Abstract
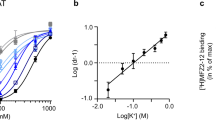
GUANINE NUCLEOTIDES have been shown to regulate the sensitivity of adenylate cyclase to hormones in several systems1–5, including the dopamine-sensitive adenylate cyclase in the caudate nucleus2,4. It has also been reported that in the presence of GTP the affinities of glucagon receptors6, β-adrenergic receptors7,8, prostaglandin E1 receptors9 and opiate receptors10 for agonists are decreased. For β-adrenergic receptors this effect of GTP has been described as ‘agonist-specific’, as it is seen with agonists but not with antagonists7,8. We report here a similar effect of GTP on the ability of dopamine-receptor agonists to compete for 3H-spiroperidol binding sites on rat striatal membranes. The presence of 0.3 mM GTP led to a four-to-fivefold increase in the Kd values for the inhibition of 3H-spiroperidol binding by dopamine-receptor agonists. No changes in Kd values were observed for antagonists. Dopamine-receptor agonists and antagonists have been defined by the stimulation or inhibition of dopamine-sensitive adenylate cyclase in the neostriatum11,12 or by their effects on the canine renal artery13. Controversy exists, however, as to whether binding studies using labelled neuroleptics such as spiroperidol actually measure dopamine receptors. Our finding of an agonist-specific effect of GTP supports the conclusion that 3H-spiroperidol is binding to functional dopamine receptors in the caudate nucleus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rodbell, M. et al. Adv. Cyclic Nucleotide Res. 5, 3–29 (1975).
Clement-Cormier, Y. C., Parrish, R. G., Petzold, G. L., Kebabian, J. W. & Greengard, P. J. J. Neurochem. 25, 143–149 (1975).
Brown, E. M., Fedak, S. A., Woodard, C. J. & Aurbach, G. D. J. biol. Chem. 251, 1239–1246 (1976).
Ronfogalis, B. D., Thornton, M. & Wade, D. N. J. Neurochem. 27, 1533–1535 (1976).
Maguire, M. E., Ross, E. M. & Gilman, A. G. Adv. Cyclic Nucleotide Res. 8, 1–83 (1977).
Rodbell, M., Krans, H. M. J., Pohl, S. L. & Birnbaumer, L. J. biol. Chem. 246, 1872–1876 (1971).
Maguire, M. E., Van Arsdale, P. M. & Gilman, A. G. Molec. Pharmac. 12, 335–339 (1976).
Williams, L. T. & Lefkowitz, R. J. J. biol. Chem. 252, 7207–7213 (1977).
Lefkowitz, R. J., Mullikin, D., Wood, C. L., Gore, T. B. & Mukherjee, C. J. biol. Chem. 252, 5295–5303 (1977).
Blume, A. J. Proc. natn. Acad. Sci. U.S.A. 75, 1713–1717 (1978).
Miller, R. J., Horn, A. S. & Iversen, L. L. Molec. Pharmac. 10, 759–766 (1974).
Iversen, L. L. Science 188, 1084–1089 (1975).
Goldberg, L. I., Volkman, P. H. & Kohli, J. D. A. Rev. Pharmac. Toxic. 18, 57–79 (1978).
Creese, I., Schneider, R. & Snyder, S. Eur. J. Pharmac. 46, 377–381 (1977).
Fields, J. Z., Reisine, T. D. & Yamamura, H. I. Brain Res. 136, 578–584 (1977).
Leysen, J. E., Gommeren, W. & Laduron, P. M. Biochem. Pharmac. 27, 307–316 (1978).
Seeman, P., Chau-Wong, M., Tedesco, J. & Wong, K. Proc. natn. Acad. Sci. U.S.A. 72, 4376–4380 (1975).
Burt, D. R., Enna, S. J., Creese, I. & Snyder, S. H. Proc. natn. Acad. Sci. U.S.A. 72, 4655–4659 (1975).
Roberts, P. J., Woodruff, G. N. & Poat, J. A. Molec. Pharmac. 13, 541–547 (1977).
Haga, R., Ross, E., Anderson, H. J. & Gilman, A. G. Proc. natn. Acad. Sci. U.S.A. 74, 2016–2020 (1977).
Kebabian, J. Brain Res. 144, 194–198 (1978).
Klee, W. A. & Nirenberg, M. Nature 263, 609–612 (1976).
Burt, D. R., Creese, I. & Snyder, S. H. Molec. Pharmac. 12, 800–812 (1976).
Laduron, P. M., Janssen, P. F. M. & Leysen, J. E. Biochem. Pharmac. 27, 323–328 (1978).
Schwarcz, R., Creese, I., Coyle, J. T. & Snyder, S. H. Nature 271, 766–768 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ZAHNISER, N., MOLINOFF, P. Effect of guanine nucleotides on striatal dopamine receptors. Nature 275, 453–455 (1978). https://doi.org/10.1038/275453a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/275453a0
This article is cited by
-
Within-Subject Comparison of [11C]-( + )-PHNO and [11C]raclopride Sensitivity to Acute Amphetamine Challenge in Healthy Humans
Journal of Cerebral Blood Flow & Metabolism (2012)
-
First Human Evidence of d-Amphetamine Induced Displacement of a D2/3 Agonist Radioligand: A [11C]-(+)-PHNO Positron Emission Tomography Study
Neuropsychopharmacology (2008)
-
On the analysis of ligand-directed signaling at G protein-coupled receptors
Naunyn-Schmiedeberg's Archives of Pharmacology (2008)
-
Quantitative autoradiographic distribution and pharmacological characterization of (3H)buspirone binding to sections from rat, bovine and marmoset brain
Journal of Neural Transmission (1989)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.