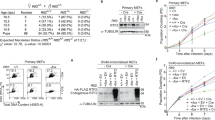
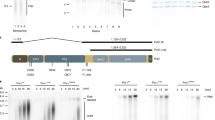
Abstract
DNA replication in eukaryotic cells initiates from many replication origins1 which fire throughout the S phase of the cell cycle in a predictable pattern: some origins fire early, others late2. Little is known about how the initiation of DNA replication and the elongation of newly synthesized DNA strands are coordinated during S phase. Here we show that, in budding yeast, hydroxyurea, which blocks the progression of replication forks from early-firing origins, also inhibits the firing of late origins. These late origins are maintained in the initiation-competent prereplicative state for extended periods. The block to late origin firing is an active process and is defective in yeast with mutations in the rad53 and mec1 checkpoint genes, indicating that regulation of late origin firing may also be an important component of the ‘intra-S-phase’ checkpoint3 and may aid cell survival under adverse conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huberman, J. A. & Riggs, A. D. On the mechanism of DNA replication in mammalian chromosomes. J. Mol. Biol. 32, 327–341 (1968).
Fangman, W. L. & Brewer, B. J. Aquestion of time–replication origins of eukaryotic chromosomes. Cell 71, 363–366 (1992).
Paulovich, A. G. & Hartwell, L. H. Acheckpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82, 841–847 (1995).
Reynolds, A. E., McCarroll, R. M., Newlon, C. S. & Fangman, W. L. Time of replication of ARS elements along yeast chromosome III. Mol. Cell. Biol. 9, 4488–4494 (1989).
Bousset, K. & Diffley, J. F. X. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 12, 480–490 (1998).
Diffley, J. F. X., Cocker, J. H., Dowell, S. J. & Rowley, A. Two steps in the assembly of complexes at yeast replication origins in vivo. Cell 78, 303–316 (1994).
Cocker, J. H., Piatti, S., Santocanale, C., Nasmyth, K. & Diffley, J. F. X. An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature 379, 180–182 (1996).
Santocanale, C. & Diffley, J. F. X. ORC- and Cdc6-dependent complexes at active and inactive chromosomal replication origins in Saccharomyces cerevisiae. EMBO J. 15, 6671–679 (1996).
Piatti, S., Bohm, T., Cocker, J. H., Diffley, J. F. X. & Nasmyth, K. Activation of S-phase promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 10, 1516–1531 (1996).
Ferguson, B. M. & Fangman, W. L. Aposition effect on the time of replication origin activation in yeast. Cell 68, 333–339 (1992).
Dubey, D. D. et al . Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol. Cell. Biol. 11, 5346–5355 (1991).
Broach, J. R. et al . Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb. Symp. Quant. Biol. 47, 1165–1173 (1982).
Elledge, S. J. Cell cycle checkpoints: preventing an identity crisis. Science 274, 1664–1672 (1996).
Raghuraman, M. K., Brewer, B. J. & Fangman, W. L. Cell cycle-dependent establishment of a late replication program. Science 276, 806–809 (1997).
Zakian, V. A. ATM-related genes: what do they tell us about functions of the human gene? Cell 82, 685–687 (1995).
Painter, R. B. & Young, B. R. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl Acad. Sci. USA 77, 7315–7317 (1980).
Larner, J. M., Lee, H. & Hamlin, J. L. Radiation effects on DNA synthesis in a defined chromosomal replicon. Mol. Cell. Biol. 14, 1901–1908 (1994).
Santocanale, C., Neecke, H., Longhese, M. P., Lucchini, G. & Plevani, P. Mutations in the gene encoding the 34 kDa subunit of yeast replication protein A cause defective S phase progression. J. Mol. Biol. 254, 595–607 (1995).
Rowley, A., Cocker, J. H., Harwood, J. & Diffley, J. F. X. Initiation complex assembly at budding yeast replication origins begins with the recognition of a bipartite sequence by limiting amounts of the initiator, ORC. EMBO J. 14, 2631–2641 (1995).
Diffley, J. F. X. & Cocker, J. H. Protein–DNA interactions at a yeast replication origin. Nature 357, 169–172 (1992).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: a Laboratory Manual (Cold Spring Harb. Lab. Press, Cold Spring Harbor, 1989).
Ferguson, B. M., Brewer, B. J., Reynolds, A. E. & Fangman, W. L. Ayeast origin of replication is activated late in S phase. Cell 65, 507–515 (1991).
Allen, J. B., Zhou, Z., Siede, W., Friedberg, E. C. & Elledge, S. J. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 8, 2401–2415 (1994).
Wellinger, R. J., Ethier, K., Labrecque, P. & Zakian, V. A. Evidence for a new step in telomere maintenance. Cell 85, 423–433 (1996).
Paulovich, A. G., Margulies, R. U., Garvik, B. M. & Hartwell, L. H. RAD9, RAD17, and RAD24 are required for S phase regulation in Saccharomyces cerevisiae in response to DNA damage. Genetics 145, 45–62 (1997).
Acknowledgements
We thank S. Elledge and L. Hartwell for yeast strains; V. A. Zakian for plasmid pYLPV; members of the Diffley lab for stimulating discussions; and members of the ICRF Photography, Peptide Synthesis and Oligonucleotide Synthesis departments for their help.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Supplementary Information
Rights and permissions
About this article
Cite this article
Santocanale, C., Diffley, J. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615–618 (1998). https://doi.org/10.1038/27001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/27001
This article is cited by
-
Cohesin maintains replication timing to suppress DNA damage on cancer genes
Nature Genetics (2023)
-
ATR kinase supports normal proliferation in the early S phase by preventing replication resource exhaustion
Nature Communications (2023)
-
Unscheduled DNA replication in G1 causes genome instability and damage signatures indicative of replication collisions
Nature Communications (2022)
-
Yeast Stn1 promotes MCM to circumvent Rad53 control of the S phase checkpoint
Current Genetics (2022)
-
H3K4 methylation at active genes mitigates transcription-replication conflicts during replication stress
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.