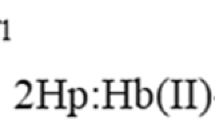Abstract
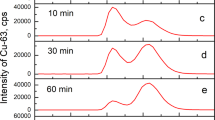
THE unloading of oxygen in the tissues is facilitated by lowering the oxygen affinity of haemoglobin (Hb) by both CO2 and diphosphoglycerate (DPG)1. This occurs because CO2 and DPG are oxygen-linked, that is, they are bound more firmly to deoxyhaemoglobin than to oxyhaemoglobin2,3. The binding of CO2 to Hb takes place by a reaction of CO2 with the α-amino groups to form carbamino compounds4,5, and binding curves can be measured by rather laborious methods4,6. Perrella et al.7 have modified these methods so that much smaller amounts of Hb, such as the specifically carbamylated Hbs8, can be used. In this method, Hb equilibrated with CO2 is rapidly taken to pH 11 to stabilise the carbamino CO2 and BioRad AG 1×8 resin added to remove carbonate and bicarbonate ions. The carbamino CO2 is displaced from the Hb by acidification and measured in a Van Slyke apparatus. DPG-free human deoxyhaemoglobin gave diphasic CO2 binding curves7 showing that the affinities of the α and β chain α-amino groups for CO2 are different, but it was impossible to establish which group had the higher affinity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Brenna, O., et al., in Advances in Experimental Medicine and Biology (edit by Brewer, G. J.), 19–37 (Plenum, New York, 1972).
Rossi-Bernardi, L., and Roughton, F. J. W., J. Physiol., Lond., 189, 1–29 (1967).
Benesch, R. E., Benesch, R., and Yu, C. I., Biochemistry, 8, 2567–2571 (1969).
Ferguson, J. K. W., and Roughton, F. J. W., J. Physiol., Lond., 83, 87–102 (1934).
Kilmartin, J. V., and Rossi-Bernardi, L., Nature, 222, 1243–1246 (1969).
Perrella, M., Rossi-Bernardi, L., and Roughton, F. J. W., in A. Benzon Symp. IV, Oxygen Affinity of Hemoglobin and Red Cell Acid Base Status (edit. by Rorth, M., and Astrup, P.), 177–203 (Munksgaard, Kobenhavn, 1971).
Perrella, M., Bresciani, D., Bresciani, D., and Rossi-Bernardi, L., J. biol. Chem. 250, 5413–5418 (1975).
Kilmartin, J. V., Fogg, J., Luzzana, M., and Rossi-Bernardi, L., J. biol. Chem., 248, 7039–7043 (1973).
Arnone, A., Nature, 237, 146–149 (1972).
Arnone, A., Nature, 247, 143–145 (1974).
Hamasaki, N., and Rose, Z. B., J. biol. Chem., 249, 7896–7901 (1974).
Berger, J., Janig, G., Gerber, G., Ruckpaul, K., and Rapoport, S. M., Eur. J. Biochem., 38, 553–562 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PERRELLA, M., KILMARTIN, J., FOGG, J. et al. Identification of the high and low affinity CO2-binding sites of human haemoglobin. Nature 256, 759–761 (1975). https://doi.org/10.1038/256759a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/256759a0
This article is cited by
-
Theoretical study of interactions between human adult hemoglobin and acetate ion by polarizable force field and fragmentation quantum chemistry methods
Science in China Series B: Chemistry (2009)
-
Comparative effects of CO2 on the affinity for O2 of fetal and adult erythrocytes
Pfl�gers Archiv European Journal of Physiology (1979)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.