Abstract
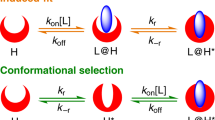
MANY of the small molecules, such as enzyme substrates and inhibitors, hormones and neurotransmitters, the interactions of which with macromolecules are of fundamental importance in biology, may exist in solution in a number of conformations in equilibrium with one another. On the other hand, evidence from crystallographic studies suggests that when bound to a macromolecule these small flexible ligands have a single, well defined, conformation. The formation of such a ligand–macromolecule complex must therefore involve a process of conformational selection, which will influence the binding constant and the kinetics of complex formation. The apparent binding constant will simply be the product of the binding constant for the ‘correct’ conformation of the ligand and the mole fraction of this conformation in solution. The kinetic effects of conformational selection will depend on the mechanism of the binding process, and two contrasting models for this may be considered1,2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Roberts, G. C. K., Proc. seventh Jerusalem Symp. Quantum Chem. Biochem. (in the press).
Feeney, J., Roberts, G. C. K., and Burgen, A. S. V., Proc. seventh Jerusalem Symp. Quantum Chem. Biochem. (in the press).
Applequist, J., and Damle, V., J. Am. Chem. Soc., 87, 1450–1458 (1965).
Porschke, D., and Eigen, M., J. molec. Biol., 62, 361–381 (1971).
Craig, M. E., Crothers, D. M., and Doty, P. M., J. molec. Biol., 62, 383–401 (1971).
Poland, D., and Scheraga, H. A., Theory of Helix-coil Transitions in Biopolymers (Academic, New York, 1970).
Engel, J., and Winklmair, D., FEBS Symp., 29, 29–44 (1972).
Laiken, N., and Nemethy, G., J. phys. Chem., 74, 4421–4441 (1970).
Partington, P., Feeney, J., and Burgen, A. S. V., Mol. Pharmacol., 8, 269–277 (1972).
Proc. seventh Jerusalem Symp. Quantum Chem. Biochem., passim., (in the press).
Meadows, D. H., Roberts, G. C. K., and Jardetzky, O., J. molec. Biol., 45, 491–511 (1969).
Richards, F. M., and Wyckoff, H. W., in The Enzymes, third ed., IV (edit. by Boyer, P. D.), (Academic, New York, 1971).
Sykes, B. D., Patt, S. L., and Dolphin, D., Cold Spring Harb. Symp. quant. Biol., 36, 29–33 (1971).
Rodgers, P., and Roberts, G. C. K., FEBS Lett., 36, 330–333 (1973).
Ganellin, C. R., J. med. Chem., 16, 620–623 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BURGEN, A., ROBERTS, G. & FEENEY, J. Binding of flexible ligands to macromolecules. Nature 253, 753–755 (1975). https://doi.org/10.1038/253753a0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1038/253753a0
This article is cited by
-
Structural changes by sulfoxidation of phenothiazine drugs
Journal of Computer-Aided Molecular Design (1992)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.