Abstract
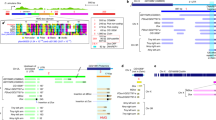
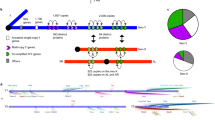
The pattern of genetic variation across the genome of Drosophila melanogaster is consistent with the occurrence of frequent ‘selective sweeps’, in which new favourable mutations become incorporated into the species so quickly that linked alleles can ‘hitchhike’ and also become fixed1. Because of the hitchhiking of linked genes, it is generally difficult to identify the target of any putative selective sweep. Here, however, we identify a new gene in D. melanogaster that codes for a sperm-specific axonemal dynein subunit. The gene has a new testes-specific promoter derived from a protein-coding region in a gene encoding the cell-adhesion protein annexin X (AnnX), and it contains a new protein-coding exon derived from an intron in a gene encoding a cytoplasmic dynein intermediate chain (Cdic). The new transcription unit, designated Sdic (for sperm-specific dynein intermediate chain), has been duplicated about tenfold in a tandem array. Consistent with the selective sweep of this gene, the level of genetic polymorphism near Sdic is unusually low. The discovery of this gene supports other results that point to the rapid molecular evolution of male reproductive functions2,3,4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Begun, D. J. & Aquadro, C. F. Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356, 519–520 (1992).
Coulthart, M. B. & Singh, R. S. High level of divergence of male-reproductive-tract proteins, between Drosophila melanogaster and its sibling species, D. simulans. Mol. Biol. Evol. 5, 182–191 (1988).
Wu, C.-I. & Davis, A. W. Evolution of postmating reproductive isolation: the composite nature of Haldane's rule and its genetic basis. Am. Nat. 142, 187–212 (1993).
Civetta, A. & Singh, R. S. Sex-related genes, directional sexual selection, and speciation. Mol. Biol. Evol. 15, 901–909 (1998).
Benevolenskaya, E. V., Nurminsky, D. I. & Gvozdev, V. A. Structure of the Drosophila melanogaster annexin X gene. DNA Cell Biol. 14, 349–357 (1994).
Nurminsky, D. I. et al. Cytoplasmic dynein intermediate chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol. Cell. Biol. 18, 6816–6825 (1998).
Steffen, W. et al. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell 8, 2077–2088 (1997).
Michiels, F., Gasch, A., Kaltschmidt, B. & Renkawitz-Pohl, R. A14 bp promoter element directs the testis specificity of the Drosophila beta 2 tubulin gene. EMBO J. 8, 1559–1565 (1989).
Petrov, D. A., Lozovskaya, E. R. & Hartl, D. L. High intrinsic rate of DNA loss in Drosophila. Nature 384, 346–349 (1996).
Moriyama, E. N. & Powell, J. R. Intraspecific nuclear DNA variation in Drosophila. Mol. Biol. Evol. 13, 261–277 (1996).
Caccone, A., Amato, G. D. & Powell, J. R. Rates and patterns of scnDNA and mtDNA divergence within the Drosophila melanogaster subgroup. Genetics 118, 671–683 (1988).
Long, M. & Langley, C. H. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science 260, 91–95 (1993).
Long, M., Rosenberg, C. & Gilbert, W. Intron phase correlations and the evolution of the intron/exon structure of genes. Proc. Natl Acad. Sci. USA 92, 12495–12499 (1995).
Nurminsky, D. I., Moriyama, E. N., Lozovskaya, E. R. & Hartl, D. L. Molecular phylogeny and genome evolution in the Drosophila virilis species group: duplication of the alcohol dehydrogenase gene. Mol. Beiol. Evol. 13, 132–149 (1996).
Patton, J. S., Gomes, X. V. & Geyer, P. K. Position-independent germline transformation in Drosophila using a cuticle pigmentation gene as a selectable marker. Nucleic Acids Res. 20, 5859–5860 (1992).
Karess, R. E. & Rubin, G. M. Analysis of P transposable element functions in Drosophila. Cell 38, 135–146 (1984).
Rubin, G. M. & Spradling, A. C. Genetic transformation of Drosophila with transposable element vectors. Science 218, 348–353 (1982).
Acknowledgements
This work was supported by grants from the US Public Health Service. We thank W.Gilbert and S. R. Palumbi for their valuable suggestions and careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nurminsky, D., Nurminskaya, M., Aguiar, D. et al. Selective sweep of a newly evolved sperm-specific gene in Drosophila. Nature 396, 572–575 (1998). https://doi.org/10.1038/25126
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25126
This article is cited by
-
Paralog transcriptional differentiation in the D. melanogaster-specific gene family Sdic across populations and spermatogenesis stages
Communications Biology (2023)
-
Evolutionary patterns of chimeric retrogenes in Oryza species
Scientific Reports (2019)
-
Living on a volcano’s edge: genetic isolation of an extremophile terrestrial metazoan
Heredity (2014)
-
Functional requirements driving the gene duplication in 12 Drosophila species
BMC Genomics (2013)
-
New genes as drivers of phenotypic evolution
Nature Reviews Genetics (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.