Abstract
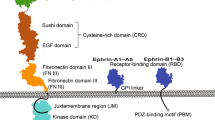
The Eph receptors, which bind a group of cell-membrane-anchored ligands known as ephrins, represent the largest subfamily of receptor tyrosine kinases (RTKs)1. They are predominantly expressed in the developing and adult nervous system2 and are important in contact-mediated axon guidance3,4,5,6, axon fasciculation5,7 and cell migration8,9,10,11. Eph receptors are unique among other RTKs in that they fall into two subclasses with distinct ligand specificities12, and in that they can themselves function as ligands to activate bidirectional cell–cell signalling4,13,14. We report here the crystal structure at 2.9 Å resolution of the amino-terminal ligand-binding domain of the EphB2 receptor (also known as Nuk)15,16,17. The domain folds into a compact jellyroll β-sandwich composed of 11 antiparallel β-strands. Using structure-based mutagenesis, we have identified an extended loop that is important for ligand binding and class specificity. This loop, which is conserved within but not between Eph RTK subclasses, packs against the concave β-sandwich surface near positions at which missense mutations cause signalling defects18, localizing the ligand-binding region on the surface of the receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands theephrins.Cell 90, 403–440 (1997).
Flanagan, J. G. & Vanderhaeghen, P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345 (1998).
Drescher, U. et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82, 359–370 (1995).
Henkemeyer, M. et al. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86, 35–46 (1996).
Orioli, D., Henkemeyer, M., Lemke, G., Klein, R. & Pawson, T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 15, 6035–6049 (1996).
Park, S., Frisen, J. & Barbacid, M. Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein receptors. EMBO J. 16, 3106–3114 (1997).
Winslow, J. W. et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron 14, 973–981 (1995).
Smith, A., Robinson, V., Patel, K. & Wilkinson, D. G. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 7, 561–570 (1997).
Xu, Q., Alldus, G., Holder, N. & Wilkinson, D. G. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development 121, 4005–4016 (1995).
Wang, H. U. & Anderson, D. J. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18, 383–396 (1997).
Krull, C. E. et al. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 7, 571–580 (1997).
Gale, N. W. et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17, 9–19 (1996).
Holland, S. J. et al. Bi-directional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383, 722–725 (1996).
Bruckner, K., Pasquale, E. B. & Klein, R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275, 1640–1643 (1997).
Henkemeyer, M. et al. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene 9, 1001–1014 (1994).
Labrador, J. P., Brambilla, R. & Klein, R. The N-terminal globular domain of Eph receptors is sufficient for ligand binding and receptor signaling. EMBO J. 16, 3889–3897 (1997).
Lackmann, M. et al. Distinct subdomains of the EphA3 receptor mediate ligand binding and receptor dimerization. J. Biol. Chem. 273, 20228–20237 (1998).
George, S. E., Simokat, K., Hardin, J. & Chisholm, A. D. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92, 633–643 (1998).
Lackmann, M. et al. Ligand for EPH-related kinase (LERK) 7 is the preferred high affinity ligand for the HEK receptor. J. Biol. Chem. 272, 16521–16530 (1997).
Rini, J. M. Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 24, 551–577 (1995).
Holm, L. & Sander, C. Touring protein fold space with Dali/FSSP. Nucleic Acids Res. 26, 316–319 (1998).
Van der Geer, P., Hunter, T. & Lindberg, R. A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 10, 251–337 (1994).
Banner, D. W. et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNFβ complex: implications for TNF receptor activation. Cell 73, 431–445 (1993).
Sauter, N. K. et al. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 31, 9609–9621 (1992).
Delbaere, L. T. et al. Structures of the lectin IV of Griffonia simplicifolia and its complex with the Lewis b human blood group determinant 2.0 Å resolution. J. Mol. Biol. 230, 950–965 (1993).
Otwinowski, Z. & Minor, W. in Data Collection and Processing (eds Sawyer, L., Isaacs, N. & Bailey, S.) 556–562 (SERC Daresbury Laboratory, Warrington, (1993)).
CCP4.The CCP4 suite: programs for x-ray crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A. T. X-PLOR v. 3.1 Manual (Yale Univ. Press, New Haven, (1993)).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Acknowledgements
We thank I. Berry for technical support; P. D. Jeffrey for help with X-ray measurements; U. Drescher for ephrin-A5 DNA; and S. K. Burley, J. Goldberg, N. P. Pavletich and M.K. Rosen for useful suggestions. J.-P.H. is a Winston Foundation fellow. This work was supported by the DeWitt Wallace Fund and the V Foundation (D.B.N.), and by the Kent Waldrep National Paralysis Foundation for Basic Neuroscience Research (M.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Himanen, JP., Henkemeyer, M. & Nikolov, D. Crystal structure of the ligand-binding domain of the receptor tyrosine kinase EphB2. Nature 396, 486–491 (1998). https://doi.org/10.1038/24904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/24904
This article is cited by
-
Protein dynamics at Eph receptor-ligand interfaces as revealed by crystallography, NMR and MD simulations
BMC Biophysics (2012)
-
Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling
Nature Neuroscience (2004)
-
Eph–ephrin promiscuity is now crystal clear
Nature Neuroscience (2004)
-
Mechanisms and functions of eph and ephrin signalling
Nature Reviews Molecular Cell Biology (2002)
-
Crystal structure of an Eph receptor–ephrin complex
Nature (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.