Abstract
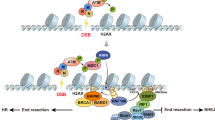
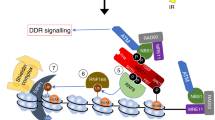
The packaging and compaction of DNA into chromatin is important for all DNA-metabolism processes such as transcription, replication and repair. The involvement of chromatin modifications in transcriptional regulation is relatively well characterized, and the distinct patterns of chromatin transitions that guide the process are thought to be the result of a code on the histone proteins (histone code). In contrast to transcription, the intricate link between chromatin and responses to DNA damage has been given attention only recently. It is now emerging that specific ATP-dependent chromatin remodeling complexes (including the Ino80, Swi/Snf and RSC remodelers) and certain constitutive (methylation of lysine 79 of histone H3) and DNA damage-induced covalent histone modifications (the most well characterized being the rapid phosphorylation of histone H2A) facilitate responses to double-strand breaks. Indeed, evidence is already accumulating for a DNA repair-specific histone code. In this review, the recent advances in our understanding of the relationship between chromatin modifications and double-strand break signaling and repair is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Jackson SP . Sensing and repairing DNA double-strand breaks. Carcinogenesis 2002; 23: 687–696.
Khanna KK, Jackson SP . DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 2001; 27: 247–254.
Lieber MR, Ma Y, Pannicke U, Schwarz K . Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 2003; 4: 712–720.
Khanna KK, Lavin MF, Jackson SP, Mulhern TD . ATM, a central controller of cellular responses to DNA damage. Cell Death Differ 2001; 8: 1052–1065.
Shiloh Y . ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003; 3: 155–168.
Cahill D, Connor B, Carney JP . Mechanisms of eukaryotic DNA double strand break repair. Front Biosci 2006; 11: 1958–1976.
O'Driscoll M, Jeggo PA . The role of double-strand break repair – insights from human genetics. Nat Rev Genet 2006; 7: 45–54.
Ahnesorg P, Smith P, Jackson SP . XLF interacts with the XRCC4–DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell 2006; 124: 301–313.
Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell 2006; 124: 287–299.
Jackson SP . Detecting, signalling and repairing DNA double-strand breaks. Biochem Soc Trans 2001; 29: 655–661.
Sonoda E, Takata M, Yamashita YM, Morrison C, Takeda S . Homologous DNA recombination in vertebrate cells. Proc Natl Acad Sci USA 2001; 98: 8388–8394.
Thompson LH, Schild D . Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutat Res 2001; 477: 131–153.
Wood RD, Mitchell M, Sgouros J, Lindahl T . Human DNA repair genes. Science 2001; 291: 1284–1289.
Arents G, Moudrianakis EN . Topography of the histone octamer surface: repeating structural motifs utilized in the docking of nucleosomal DNA. Proc Natl Acad Sci USA 1993; 90: 10489–10493.
Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ . Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997; 389: 251–260.
Horn PJ, Peterson CL . Molecular biology. Chromatin higher order folding – wrapping up transcription. Science 2002; 297: 1824–1827.
Kamakaka RT, Biggins S . Histone variants: deviants? Genes Dev 2005; 19: 295–310.
Saha A, Wittmeyer J, Cairns BR . Chromatin remodeling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol 2006; 7: 437–447.
Iizuka M, Smith MM . Functional consequences of histone modifications. Curr Opin Genet Dev 2003; 13: 154–160.
Spotswood HT, Turner BM . An increasingly complex code. J Clin Invest 2002; 110: 577–582.
Bakkenist CJ, Kastan MB . DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506.
Kruhlak MJ, Celeste A, Dellaire G, Fernandez-Capetillo O, Muller WG, McNally JG et al. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol 2006; 172: 823–834.
Cerosaletti K, Wright J, Concannon P . Active role for nibrin in the kinetics of atm activation. Mol Cell Biol 2006; 26: 1691–1699.
Lee JH, Paull TT . Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 2004; 304: 93–96.
Lee JH, Paull TT . ATM activation by DNA double-strand breaks through the Mre11–Rad50–Nbs1 complex. Science 2005; 308: 551–554.
Falck J, Coates J, Jackson SP . Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005; 434: 605–611.
Rogakou EP, Boon C, Redon C, Bonner WM . Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146: 905–916.
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A . H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004; 3: 959–967.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10: 886–895.
Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature 2003; 421: 952–956.
Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ . MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 2003; 421: 961–966.
Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP . MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 2005; 123: 1213–1226.
Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5: 675–679.
Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA et al. Genomic instability in mice lacking histone H2AX. Science 2002; 296: 922–927.
Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM . Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol 2003; 81: 123–129.
Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA . ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 2004; 64: 2390–2396.
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ . ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276: 42462–42467.
Ward IM, Chen J . Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276: 47759–47762.
Vidanes GM, Bonilla CY, Toczyski DP . Complicated tails: histone modifications and the DNA damage response. Cell 2005; 121: 973–976.
Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE et al. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol Cell 2004; 16: 991–1002.
Downs JA, Lowndes NF, Jackson SP . A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 2000; 408: 1001–1004.
Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH et al. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol 2004; 14: 1703–1711.
Madigan JP, Chotkowski HL, Glaser RL . DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res 2002; 30: 3698–3705.
Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates III JR et al. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 2004; 306: 2084–2087.
van Attikum H, Gasser SM . The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol 2005; 6: 757–765.
Xie A, Puget N, Shim I, Odate S, Jarzyna I, Bassing CH et al. Control of sister chromatid recombination by histone H2AX. Mol Cell 2004; 16: 1017–1025.
Strom L, Lindroos HB, Shirahige K, Sjogren C . Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol Cell 2004; 16: 1003–1015.
Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J . gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 2005; 20: 801–809.
Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 2006; 439: 497–501.
van Attikum H, Gasser SM . ATP-dependent chromatin remodeling and DNA double-strand break repair. Cell Cycle 2005; 4: 1011–1014.
Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 2004; 119: 767–775.
van Attikum H, Fritsch O, Hohn B, Gasser SM . Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004; 119: 777–788.
Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N et al. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 2004; 16: 979–990.
Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 2002; 419: 411–415.
Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C . ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 2004; 303: 343–348.
Shen X, Mizuguchi G, Hamiche A, Wu C . A chromatin remodelling complex involved in transcription and DNA processing. Nature 2000; 406: 541–544.
Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ et al. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2AZ into euchromatin. PLoS Biol 2004; 2: E131.
Shim EY, Ma JL, Oum JH, Yanez Y, Lee SE . The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks. Mol Cell Biol 2005; 25: 3934–3944.
Chai B, Huang J, Cairns BR, Laurent BC . Distinct roles for the RSC and Swi/Snf ATP-dependent chromatin remodelers in DNA double-strand break repair. Genes Dev 2005; 19: 1656–1661.
Bennett CB, Lewis LK, Karthikeyan G, Lobachev KS, Jin YH, Sterling JF et al. Genes required for ionizing radiation resistance in yeast. Nat Genet 2001; 29: 426–434.
Koyama H, Itoh M, Miyahara K, Tsuchiya E . Abundance of the RSC nucleosome-remodeling complex is important for the cells to tolerate DNA damage in Saccharomyces cerevisiae. FEBS Lett 2002; 531: 215–221.
Wolner B, Peterson CL . ATP-dependent and ATP-independent roles for the Rad54 chromatin remodeling enzyme during recombinational repair of a DNA double strand break. J Biol Chem 2005; 280: 10855–10860.
Jaskelioff M, Van Komen S, Krebs JE, Sung P, Peterson CL . Rad54p is a chromatin remodeling enzyme required for heteroduplex DNA joint formation with chromatin. J Biol Chem 2003; 278: 9212–9218.
Alexeev A, Mazin A, Kowalczykowski SC . Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51–ssDNA nucleoprotein filament. Nat Struct Biol 2003; 10: 182–186.
Alexiadis V, Kadonaga JT . Strand pairing by Rad54 and Rad51 is enhanced by chromatin. Genes Dev 2002; 16: 2767–2771.
Mazin AV, Alexeev AA, Kowalczykowski SC . A novel function of Rad54 protein. Stabilization of the Rad51 nucleoprotein filament. J Biol Chem 2003; 278: 14029–14036.
Choy JS, Kron SJ . NuA4 subunit Yng2 function in intra-S-phase DNA damage response. Mol Cell Biol 2002; 22: 8215–8225.
Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol 2006; 8: 91–99.
Jazayeri A, McAinsh AD, Jackson SP . Saccharomyces cerevisiae Sin3p facilitates DNA double-strand break repair. Proc Natl Acad Sci USA 2004; 101: 1644–1649.
Cheung WL, Turner FB, Krishnamoorthy T, Wolner B, Ahn SH, Foley M et al. Phosphorylation of histone H4 serine 1 during DNA damage requires casein kinase II in S. cerevisiae. Curr Biol 2005; 15: 656–660.
Fernandez-Capetillo O, Allis CD, Nussenzweig A . Phosphorylation of histone H2B at DNA double-strand breaks. J Exp Med 2004; 199: 1671–1677.
Huyen Y, Zgheib O, Ditullio Jr RA, Gorgoulis VG, Zacharatos P, Petty TJ et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 2004; 432: 406–411.
Weinert TA, Hartwell LH . The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 1988; 241: 317–322.
Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M . Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes Dev 1997; 11: 3387–3400.
Giannattasio M, Lazzaro F, Plevani P, Muzi-Falconi M . The DNA damage checkpoint response requires histone H2B ubiquitination by Rad6-Bre1 and H3 methylation by Dot1. J Biol Chem 2005; 280: 9879–9886.
Briggs SD, Xiao T, Sun ZW, Caldwell JA, Shabanowitz J, Hunt DF et al. Gene silencing: trans-histone regulatory pathway in chromatin. Nature 2002; 418: 498.
Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T . Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 2004; 119: 603–614.
Nakamura TM, Du LL, Redon C, Russell P . Histone H2A phosphorylation controls Crb2 recruitment at DNA breaks, maintains checkpoint arrest, and influences DNA repair in fission yeast. Mol Cell Biol 2004; 24: 6215–6230.
Du LL, Nakamura TM, Russell P . Histone modification-dependent and -independent pathways for recruitment of checkpoint protein Crb2 to double-strand breaks. Genes Dev 2006; 20: 1583–1596.
Acknowledgements
We acknowledge the support of the Australian Institute of Nuclear Science and Engineering. TCK was the recipient of AINSE awards. Molecular Radiation Biology and Epigenetics in Human Health and Disease Laboratories are supported by the National Health and Medical Research Council of Australia (350359 and 268905).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karagiannis, T., El-Osta, A. Chromatin modifications and DNA double-strand breaks: the current state of play. Leukemia 21, 195–200 (2007). https://doi.org/10.1038/sj.leu.2404478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404478
Keywords
This article is cited by
-
Nutritional epigenomic and DNA-damage modulation effect of natural stilbenoids
Scientific Reports (2023)
-
Abnormal Histones Acetylation in Patients with Primary Sjögren's Syndrome
Clinical Rheumatology (2022)
-
Processing of DNA double strand breaks by alternative non-homologous end-joining in hyperacetylated chromatin
Genome Integrity (2012)
-
Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions
Nature (2009)
-
Chromosome shattering: a mitotic catastrophe due to chromosome condensation failure
European Biophysics Journal (2009)