Abstract
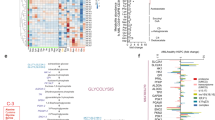
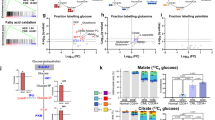
The cells of solid tumours are known to have an altered metabolism, with high rates of glucose uptake and glycolysis, which results in the excessive production of lactate. To date there has been no definitive research documenting metabolic changes in acute lymphoblastic leukaemia (ALL) cells. In order to investigate whether ALL cells have an altered metabolism, we initially compared the transcriptional profiles of 22 specimens from paediatric patients diagnosed with ALL to five CD34+ specimens isolated from bone marrow, which was verified in an independent cohort of 101 specimens. Profiling revealed the upregulation of genes facilitating glycolysis in the ALL specimens compared to the CD34+ specimens, while those involved in the tricarboxylic acid cycle were downregulated. Functional studies supported the microarray findings threefold: (1) higher expression of the glucose transport protein glucose transporter 1 in ALL compared to CD34+ specimens, (2) the excessive production of lactate in ALL cell lines and (3) sensitivity of ALL cell lines to the glycolysis inhibitor 2-deoxy-D-glucose. While metabolic alterations have been well documented in solid tumours, this is the first study to provide direct evidence for the existence of metabolic changes in the leukaemic cells of ALL patients. The finding offers new options for targeted therapy for ALL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Warburg O . On the origin of cancer cells. Science 1956; 123: 309–314.
John AP . Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Med Hypotheses 2001; 57: 429–431.
Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia 2000; 14: 2223–2233.
Kees UR, Ford J, Watson M, Murch A, Ringner M, Walker RL et al. Gene expression profiles in a panel of childhood leukemia cell lines mirror critical features of the disease. Mol Cancer Ther 2003; 2: 671–677.
Hoffmann K, Firth MJ, Freitas JR, de Klerk NH, Kees UR . Gene expression levels in small specimens from patients detected using oligonucleotide arrays. Mol Biotechnol 2005; 29: 31–38.
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP . Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 2003; 31: e15.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4: 249–264.
Ross ME, Zhou X, Song G, Shurtleff SA, Girtman K, Williams WK et al. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood 2003; 102: 2951–2959.
Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 2004; 101: 6062–6067.
Greene WK, Ford J, Dixon D, Tilbrook PA, Watt PM, Klinken SP et al. Enforced expression of HOX11 is associated with an immature phenotype in J2E erythroid cells. Br J Haematol 2002; 118: 909–917.
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988; 48: 589–601.
Chandramouli V, Carter Jr JR . Metabolic effects of 2-deoxy-D-glucose in isolated fat cells. Biochim Biophys Acta 1977; 496: 278–291.
Wu R . The effect of azide and oligomycin on inorganic phosphate transport in slices of rat kidney. Biochimica et Biophysica Acta 1964; 82: 212–215.
Onetti R, Baulida J, Bassols A . Increased glucose transport in ras-transformed fibroblasts: a possible role for N-glycosylation of GLUT1. FEBS Lett 1997; 407: 267–270.
Ahmed N, Berridge MV . N-glycosylation of glucose transporter-1 (Glut-1) is associated with increased transporter affinity for glucose in human leukemic cells. Leuk Res 1999; 23: 395–401.
Miccheli A, Tomassini A, Puccetti C, Valerio M, Peluso G, Tuccillo F et al. Metabolic profiling by (13)C-NMR spectroscopy: [1,2-(13)C(2)]glucose reveals a heterogeneous metabolism in human leukemia T cells. Biochimie 2006; 88: 437–448.
dos Santos MA, Borges JB, de Almeida DC, Curi R . Metabolism of the microregions of human breast cancer. Cancer Lett 2004; 216: 243–248.
Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P . Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia 2005; 19: 2153–2158.
Tiefenthaler M, Amberger A, Bacher N, Hartmann BL, Margreiter R, Kofler R et al. Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br J Haematol 2001; 114: 574–580.
Sillos EM, Shenep JL, Burghen GA, Pui CH, Behm FG, Sandlund JT . Lactic acidosis: a metabolic complication of hematologic malignancies: case report and review of the literature. Cancer 2001; 92: 2237–2246.
Oskam R, Rijksen G, Staal GE, Vora S . Isozymic composition and regulatory properties of phosphofructokinase from well-differentiated and anaplastic medullary thyroid carcinomas of the rat. Cancer Res 1985; 45: 135–142.
Staal GE, Kalff A, Heesbeen EC, van Veelen CW, Rijksen G . Subunit composition, regulatory properties, and phosphorylation of phosphofructokinase from human gliomas. Cancer Res 1987; 47: 5047–5051.
Gatenby RA, Gillies RJ . Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891–899.
Harris AL . Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47.
Miceli MV, Jazwinski SM . Common and cell type-specific responses of human cells to mitochondrial dysfunction. Exp Cell Res 2005; 302: 270–280.
Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A . Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 2001; 276: 9519–9525.
Dang CV, Semenza GL . Oncogenic alterations of metabolism. Trends Biochem Sci 1999; 24: 68–72.
Noguchi Y, Marat D, Saito A, Yoshikawa T, Doi C, Fukuzawa K et al. Expression of facilitative glucose transporters in gastric tumors. Hepatogastroenterology 1999; 46: 2683–2689.
Selak MA, Armour SM, Mackenzie ED, Boulahbel H, Watson DG, Mansfield KD et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005; 7: 77–85.
Habano W, Sugai T, Nakamura S, Uesugi N, Higuchi T, Terashima M et al. Reduced expression and loss of heterozygosity of the SDHD gene in colorectal and gastric cancer. Oncol Rep 2003; 10: 1375–1380.
Wellmann S, Guschmann M, Griethe W, Eckert C, von Stackelberg A, Lottaz C et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia 2004; 18: 926–933.
Lugthart S, Cheok MH, den Boer ML, Yang W, Holleman A, Cheng C et al. Identification of genes associated with chemotherapy crossresistance and treatment response in childhood acute lymphoblastic leukemia. Cancer Cell 2005; 7: 375–386.
Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med 2004; 351: 533–542.
Acknowledgements
We would like to thank Dr David Baker, Dr Nicholas Gottardo and staff of the Princess Margaret Hospital, Perth for their invaluable support, as well as the patients and parents involved in this study. We are grateful to Dr Lawrence Abraham for his critical evaluation of the manuscript. This work was supported by the National Health and Medical Research Council and the Children's Leukaemia and Cancer Research Foundation, Perth, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Boag, J., Beesley, A., Firth, M. et al. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia 20, 1731–1737 (2006). https://doi.org/10.1038/sj.leu.2404365
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404365
Keywords
This article is cited by
-
Comparison of the blood, bone marrow, and cerebrospinal fluid metabolomes in children with b-cell acute lymphoblastic leukemia
Scientific Reports (2021)
-
Pyrvinium Pamoate Use in a B cell Acute Lymphoblastic Leukemia Model of the Bone Tumor Microenvironment
Pharmaceutical Research (2020)
-
Metabolic reprogramming of acute lymphoblastic leukemia cells in response to glucocorticoid treatment
Cell Death & Disease (2018)
-
The Lipid Side of Bone Marrow Adipocytes: How Tumor Cells Adapt and Survive in Bone
Current Osteoporosis Reports (2018)
-
Differentiation resistance through altered retinoblastoma protein function in acute lymphoblastic leukemia: in silico modeling of the deregulations in the G1/S restriction point pathway
BMC Systems Biology (2016)