Abstract
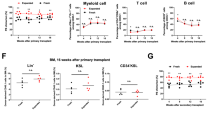
We have been investigating the hematopoietic stem cell (HSC) activity of peripheral blood-derived CD34+ cells selected by two different laboratory immunomagnetic beads systems (MiniMACS and Isolex 50). In this study, the quality of purified CD34+ cells was directly compared using clonal cell culture, a cobblestone area-forming cell (CAFC) assay, and an in vivo severe combined immunodeficiency (SCID)-repopulating cell (SRC) assay. It was found that CD34+ cells selected by these two immunomagnetic methods showed a reduced yield of colony-forming cells and CAFCs compared with cells enriched by the StemSep device (a negative selection method). However, these CD34+ cells still showed significant SRC activity, including multilineage lymphomyeloid reconstitution. The percentage of human CD45+ cells in murine bone marrow after transplanting 5 × 105 CD34+ cells selected by the Isolex 50 was significantly lower than after transplanting cells selected by the MiniMACS or the StemSep. Our findings clearly demonstrated that CD34+ cells selected by the MiniMACS system had superior HSC functions, including SRC activity, compared with cells separated by the Isolex 50 system. More detailed functional analysis of immunomagnetically separated CD34+ cells may provide useful knowledge for basic research on HSCs as well as for clinical HSC transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Krause DS, Fackler MJ, Civin CI, May WS . CD34. Structure, biology, and clinical utility. Blood 1996; 87: 1–13.
Vogel W, Scheding S, Kanz L, Brugger W . Clinical applications of CD34+ peripheral blood progenitor cells (PBPC). Stem Cells 2000; 18: 87–92.
Huntenburg CC, Kunkel LA, Schneidkraut MJ . CD34+ cell engraftment, ex vivo expansion, and malignant cell depletion following immunomagnetic selection. J Hematother 1998; 7: 175–183.
Voso MT, Hohaus S, Moos M, Pforsich M, Cremer FW, Schlenk RF et al. Autografting with CD34+ peripheral blood stem cells: retained engrafment capability and reduced tumor cell content. Br J Haematol 1999; 104: 382–391.
Mapara MY, Korner IJ, Hildebrandt M, Bargou R, Krahl D, Reichardt P et al. Monitoring of tumor cell purging after highly efficient immunomagnetic selection of CD34 cells from leukapheresis products in breast cancer patients: comparison of immunocytochemical tumor cell staining and reverse transcriptase-polymerase chain reaction. Blood 1997; 89: 337–344.
Dreger P, Viehmann K, von Neuhoff N, Kruss D, Glass B, Kneba M et al. A prospective study of positive/negative ex vivo B-cell depletion in patients with chronic lymphocytic leukemia. Exp Hematol 2000; 28: 1187–1196.
Burgess J, Mills B, Griffith M, Mansour V, Weaver CH, Schwartzberg LS et al. Breast tumor contamination of PBSC harvests: tumor depletion by positive selection of CD34+ cells. Cytotherapy 2001; 3: 285–294.
Lane TA, Law P, Maruyama M, Young D, Burgess J, Mullen M et al. Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte–macrophage colony-stimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood 1995; 85: 275–282.
Williams SF, Lee WJ, Bender JG, Zimmerman T, Swinney P, Blake M et al. Selection and expansion of peripheral blood CD34+ cells in autologous stem cell transplantation for breast cancer. Blood 1996; 87: 1687–1691.
Blystad AK, Holte H, Kvaloy S, Smeland E, Delabie J, Kvalheim G . High-dose therapy in patients with Hodgkin's disease: the use of selected CD34+ cells is as safe as unmanipulated peripheral blood progenitor cells. Bone Marrow Transplant 2001; 28: 849–857.
Klein JL, Hamm C, Dansey RD, Karanes C, Abella E, Cassells L et al. High-dose chemotherapy and CD34-selected peripheral blood progenitor cell transplantation for patients with breast cancer metastatic to bone and/or bone marrow. Bone Marrow Transplant 2001; 28: 1023–1029.
Prince HM, Bashford J, Wall D, Rischin D, Parker N, Toner GC et al. Isolex 300i CD34-selected cells to support multiple cycles of high-dose therapy. Cytotherapy 2002; 4: 137–145.
Schumm M, Lang P, Taylor G, Kuci S, Klingebiel T, Buhring HJ et al. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. J Hematother 1999; 8: 209–218.
Despres D, Flohr T, Uppenkamp M, Baldus M, Hoffmann M, Huber C et al. CD34+ cell enrichment for autologous peripheral blood stem cell transplantation by use of the CliniMACS device. J Hematother Stem Cell Res 2000; 9: 557–564.
Rosen O, Thiel A, Massenkeil G, Hiepe F, Haupl T, Radtke H et al. Autologous stem-cell transplantation in refractory autoimmune diseases after in vivo immunoablation and ex vivo depletion of mononuclear cells. Arthritis Res 2000; 2: 327–336.
Laurenti L, Sora F, Piccirillo N, Chiusolo P, Cicconi S, Rutella S et al. Immune reconstitution after autologous selected peripheral blood progenitor cell transplantation: comparison of two CD34+ cell-selection systems. Transfusion 2001; 41: 783–789.
Prince HM, Wall D, Rischin D, Toner GC, Seymour JF, Blakey D et al. CliniMACS CD34-selected cells to support multiple cycles of high-dose therapy. Cytotherapy 2002; 4: 147–155.
Larochelle A, Vormoor J, Hanenberg H, Wang JCY, Bhatia M, Lapidot T et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med 1996; 2: 1329–1337.
Kuzuyama Y, Sonoda Y, Sakabe H, Nakagawa H, Fujii H, Abe T . Successful collection of peripheral blood stem cells mobilized by a combination of G-CSF with high-dose Ara-C or high-dose etoposide. Jpn J Clin Hematol 1993; 34: 1525–1531.
Takaue Y, Watanabe T, Kawano Y, Koyama T, Abe T, Suzue T et al. Isolation and storage of peripheral blood hematopoietic stem cells for autotransplantation into children with cancer. Blood 1989; 74: 1245–1251.
Minamiguchi H, Kimura T, Urata Y, Miyazaki H, Bamba T, Abe T et al. Simultaneous signalling through c-mpl, c-kit, and CXCR4 enhances the proliferation and differentiation of human megakaryocyte progenitors: possible roles of the PI3-K, PKC and MAPK pathways. Br J Haematol 2001; 115: 175–185.
Philpott NJ, Turner AJC, Scopes J, Westby M, Marsh JCW, Gordon-Smith EC et al. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood 1996; 87: 2244–2251.
Kimura T, Sakabe H, Tanimukai S, Abe T, Urata Y, Yasukawa K et al. Simultaneous activation of signals through gp130, c-kit, and interleukin-3 receptor promotes a trilineage blood cell production in the absence of terminally acting lineage-specific factors. Blood 1997; 90: 4767–4778.
Sonoda Y, Sakabe H, Ohmisono Y, Tanimukai S, Yokota S, Nakagawa S et al. Synergistic actions of stem cell factor and other burst-promoting activities on proliferation of CD34+ highly purified blood progenitors expressing HLA-DR or different levels of c-kit protein. Blood 1994; 84: 4099–4106.
Ohmizono Y, Sakabe H, Kimura T, Tanimukai S, Matsumura T, Miyazaki H et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia 1997; 11: 524–530.
Tanimukai S, Kimura T, Skabe H, Ohmizono Y, Kato T, Miyazaki H et al. Recombinant human c-Mpl ligand (thrombopoietin) not only acts on megakaryocyte progenitors, but also on erythroid and multipotential progenitors in vitro. Exp Hematol 1997; 25: 1025–1033.
Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol 1989; 17: 145–153.
Sakabe H, Yahata N, Kimura T, Zeng ZZ, Minamiguchi H, Kaneko H et al. Human cord blood-derived primitive progenitors are enriched in CD34+c-kit− cells: correlation between long-term culture-initiating cells and telomerase expression. Leukemia 1998; 12: 728–734.
Breems DA, Blockland EAW, Neben S, Ploemacher RE . Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia 1994; 8: 1095–1104.
Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE . A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med 1998; 4: 1038–1045.
Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y et al. SCID-repopulating cell activity of human cord blood-derived CD34-negative cells assured by intra-bone marrow injection. Blood 2003; 101: 2924–2931.
Kaneko H, Kimura T, Tsuda S, Ohkawara Y, Abe T, Sonoda Y . CD34-positive cell selection using an Isolex 300 system in patients with solid tumors and its application for autologous peripheral blood stem cell transplantation. Jpn J Clin Hematol 1998; 39: 652–657.
Sakabe H, Ohmizono Y, Tanimukai S, Kimura T, Mori KJ, Abe T et al. Functional differences between subpopulations of mobilized peripheral blood-derived CD34+ cells expressing different levels of HLA-DR, CD33, CD38, and c-kit antigens. Stem Cells 1997; 15: 73–81.
Osawa M, Hanada K, Hamada H, Nakauchi H . Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 1996; 273: 242–245.
Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M . Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol 1998; 26: 353–360.
Kato S, Ando K, Nakamura Y, Muguruma Y, Sato T, Yabe H et al. Absence of a CD34− hematopoietic precursor population in recipients of CD34+ stem cell transplantation. Bone Marrow Transplant 2001; 28: 587–595.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research on Priority areas (Grant No. 15039227) and for Scientific Research C (Grant No. 15591015) from the Ministry of Education, Science and Culture of Japan, and Japan Leukemia Research Foundation. We are grateful to Dr Hikari Nishigaki of Ohtsu Municipal Hospital for providing a sample for this study. We also thank Kirin Brewery Co., Ltd for providing the various growth factors used in this study. Furthermore, we also wish to thank Dr Ohtsura Niwa of Radiation Biology Center, Kyoto University for his advice on the irradiation of NOD/SCID mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, T., Minamiguchi, H., Wang, J. et al. Impaired stem cell function of CD34+ cells selected by two different immunomagnetic beads systems. Leukemia 18, 566–574 (2004). https://doi.org/10.1038/sj.leu.2403211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403211
Keywords
This article is cited by
-
CD16 antigen is a positive marker of peripheral blood-derived early endothelial progenitor cells
International Journal of Hematology (2011)
-
In vivo dynamics of human cord blood-derived CD34− SCID-repopulating cells using intra-bone marrow injection
Leukemia (2010)
-
Ex vivo expansion and long-term hematopoietic reconstitution ability of sorted CD34+CD59+ cells from patients with paroxysmal nocturnal hemoglobinuria
International Journal of Hematology (2010)