Abstract
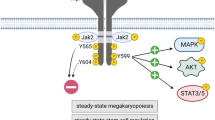
Thrombopoietin (TPO) and its receptor (MPL) are important regulators of megakaryopoiesis. MPL belongs to a cytokine receptor superfamily. To date, all constitutively active MPL mutants have been artificially constructed with amino acid substitutions in the transmembrane domain or extracellular domain of the protein, and they activate signal transduction pathways in Ba/F3 cells that can also be activated by the normal MPL. In this paper, we report a novel spontaneously occurring mutation of MPL, with an amino acid substitution of Trp508 to Ser508 in the intracellular domain of MPL, that induces the factor-independent growth of Ba/F3 cells. Examination of intracellular signaling pathways demonstrated that the mutant MPL protein constitutively activates three distinct signaling pathways, SHC-Ras-Raf-MAPK/JNK, JAK-STAT, and PI3K-Akt-Bad.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hoffman R, Long MW . Control of thrombocytopoiesis: current state of the art Cancer Treat Res 1995 80: 25–49
Kaushansky K . Thrombopoietin: the primary regulator of platelet production Blood 1995 86: 419–431
Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P . Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional Blood 1995 85: 402–413
Debili N, Wendling F, Katz A, Guichard J, Breton-Gorius J, Hunt P, Vainchenker W . The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors Blood 1995 86: 2516–2525
Kaushansky K, Broudy VC, Lin N, Jorgensen MJ, McCarty J, Fox N, Zucker-Franklin D, Lofton-Day C . Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development Proc Natl Acad Sci USA 1995 92: 3234–3238
Ku H, Yonemura Y, Kaushansky K, Ogawa M . Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice Blood 1996 87: 4544–4551
Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS, Kaushansky K . The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells Blood 1996 87: 4998–5005
Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D . Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl Blood 1996 87: 2162–2170
Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW . Thrombocytopenia in c-mpl-deficient mice Science 1994 265: 1445–1447
Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ, Eaton DL . Role of c-mpl in early hematopoiesis Blood 1998 92: 4–10
Alexander WS, Maurer AB, Novak U, Harrison-Smith M . Tyrosine-599 of the c-Mpl receptor is required for Shc phosphorylation and the induction of cellular differentiation EMBO J 1996 15: 6531–6540
Drachman JG, Griffin JD, Kaushansky K . The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-Mpl J Biol Chem 1995 270: 4979–4982
Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, Ikeda Y . Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets Blood 1996 87: 439–446
Sasaki K, Odai H, Hanazono Y, Ueno H, Ogawa S, Langdon WY, Tanaka T, Miyagawa K, Mitani K, Yazaki Y . TPO/c-mpl ligand induces tyrosine phosphorylation of multiple cellular proteins including proto-oncogene products, Vav and c-Cbl, and Ras signaling molecules Biochem Biophys Res Commun 1995 216: 338–347
Drachman JG, Millett KM, Kaushansky K . Thrombopoietin signal transduction requires functional JAK2, not TYK2 J Biol Chem 1999 274: 13480–13484
Dorsch M, Fan PD, Danial NN, Rothman PB, Goff SP . The thrombopoietin receptor can mediate proliferation without activation of the Jak-STAT pathway J Exp Med 1997 186: 1947–1955
Rouyez MC, Boucheron C, Gisselbrecht S, Dusanter-Fourt I, Porteu F . Control of thrombopoietin-induced megakaryocytic differentiation by the mitogen-activated protein kinase pathway Mol Cell Biol 1997 17: 4991–5000
Rojnuckarin P, Drachman JG, Kaushansky K . Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis Blood 1999 94: 1273–1282
Kunitama M, Shimizu R, Yamada M, Kato T, Miyazaki H, Okada K, Miura Y, Komatsu N . Protein kinase C and c-myc gene activation pathways in thrombopoietin signal transduction Biochem Biophys Res Commun 1997 231: 290–294
Hong Y, Dumenil D, van der Loo B, Goncalves F, Vainchenker W, Erusalimsky JD . Protein kinase C mediates the mitogenic action of thrombopoietin in c-Mpl-expressing UT-7 cells Blood 1998 91: 813–822
Sattler M, Salgia R, Durstin MA, Prasad KV, Griffin JD . Thrombopoietin induces activation of the phosphatidylinositol-3′ kinase pathway and formation of a complex containing p85PI3K and the protooncoprotein p120CBL J Cell Physiol 1997 171: 28–33
Ritchie A, Braun SE, He J, Broxmeyer HE . Thrombopoietin-induced conformational change in p53 lies downstream of the p44/p42 mitogen activated protein kinase cascade in the human growth factor-dependent cell line M07e Oncogene 1999 18: 1465–1477
Drachman JG, Kaushansky K . Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain Proc Natl Acad Sci USA 1997 94: 2350–2355
Luoh SM, Stefanich E, Solar G, Steinmetz H, Lipari T, Pestina TI, Jackson CW, de Sauvage FJ . Role of the distal half of the c-Mpl intracellular domain in control of platelet production by thrombopoietin in vivo Mol Cell Biol 2000 20: 507–515
del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G . Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt Science 1997 278: 687–689
Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF . Interleukin 3-dependent survival by the Akt protein kinase Proc Natl Acad Sci USA 1997 94: 11345–11350
Haseyama Y, Sawada K, Oda A, Koizumi K, Takano H, Tarumi T, Nishio M, Handa M, Ikeda Y, Koike T . Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis Blood 1999 94: 1568–1577
Bao H, Jacobs-Helber SM, Lawson AE, Penta K, Wickrema A, Sawyer ST . Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood 1999 93: 3557–3573
Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML . Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways J Biol Chem 1999 274: 23013–23019
Craddock BL, Orchiston EA, Hinton HJ, Welham MJ . Dissociation of apoptosis from proliferation, protein kinase B activation, and BAD phosphorylation in interleukin-3-mediated phosphoinositide 3-kinase signaling J Biol Chem 1999 274: 10633–10640
Miyakawa Y, Rojnuckarin P, Habib T, Kaushansky K . Thrombopoietin induces phosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes J Biol Chem 2001 276: 2494–2502
Yoshimura A, Longmore G, Lodish HF . Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity Nature 1990 348: 647–649
Jenkins BJ, D'Andrea R, Gonda TJ . Activating point mutations in the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors suggest the involvement of beta subunit dimerization and cell type-specific molecules in signalling EMBO J 1995 14: 4276–4287
Abe M, Yano S, Sakaba N, Kitamura K, Urasaki T, Nakada S, Kawasaki H, Morimoto C, Masuho Y . Surrogate thrombopoietin Immunol Lett 1998 61: 73–78
Ahmed N, Berridge MV . Distinct regulation of glucose transport by interleukin-3 and oncogenes in a murine bone marrow-derived cell line Biochem Pharmacol 1999 57: 387–396
Alexander WS, Metcalf D, Dunn AR . Point mutations within a dimer interface homology domain of c-Mpl induce constitutive receptor activity and tumorigenicity EMBO J 1995 14: 5569–5578
Onishi M, Mui AL, Morikawa Y, Cho L, Kinoshita S, Nolan GP, Gorman DM, Miyajima A, Kitamura T . Identification of an oncogenic form of the thrombopoietin receptor MPL using retrovirus-mediated gene transfer Blood 1996 88: 1399–1406
Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM . Efficiency of signalling through cytokine receptors depends critically on receptor orientation Nature 1998 395: 511–516
Otto KG, Broudy VC, Lin NL, Parganas E, Luthi JN, Drachman JG, Ihle JN, Blau CA . Membrane localization is not required for Mpl function in normal hematopoietic cells Blood 2001 98: 2077–2083
Dorsch M, Danial NN, Rothman PB, Goff SP . A thrombopoietin receptor mutant deficient in Jak-STAT activation mediates proliferation but not differentiation in UT-7 cells Blood 1999 94: 2676–2685
Miyakawa Y, Drachman JG, Gallis B, Kaushansky A, Kaushansky K . A structure–function analysis of serine/threonine phosphorylation of the thrombopoietin receptor, c-Mpl J Biol Chem 2000 275: 32214–32219
Stomski FC, Dottore M, Winnall W, Guthridge MA, Woodcock J, Bagley CJ, Thomas DT, Andrews RK, Berndt MC, Lopez AF . Identification of a 14–3-3 binding sequence in the common beta chain of the granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors that is serine-phosphorylated by GM-CSF Blood 1999 94: 1933–1942
Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G, Leonard WJ . Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I Science 1995 269: 79–81
Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM . Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor Nature 1996 379: 645–648
Kitamura T, Onishi M, Yahata T, Kanakura Y, Asano S . Activating mutations of the transmembrane domain of MPL in vitro and in vivo: incorrect sequence of MPL-K, an alternative spliced form of MPL Blood 1998 92: 2596–2597
Acknowledgements
We thank Dr Takeshi Watanabe (Kyushu University) and Dr Daisuke Kitamura (Science University of Tokyo) for their helpful comments.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abe, M., Suzuki, K., Inagaki, O. et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 16, 1500–1506 (2002). https://doi.org/10.1038/sj.leu.2402554
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402554
Keywords
This article is cited by
-
Assessing the thrombotic risk of patients with essential thrombocythemia in the genomic era
Leukemia (2017)
-
Oncogenic activation of MPL/thrombopoietin receptor by 17 mutations at W515: implications for myeloproliferative neoplasms
Leukemia (2016)
-
p22phox-dependent NADPH oxidase activity is required for megakaryocytic differentiation
Cell Death & Differentiation (2010)
-
New mutations of MPL in primitive myelofibrosis: only the MPL W515 mutations promote a G1/S-phase transition
Leukemia (2008)
-
Requirement for the PI3K/Akt pathway in MEK1-mediated growth and prevention of apoptosis: identification of an Achilles heel in leukemia
Leukemia (2003)