Abstract
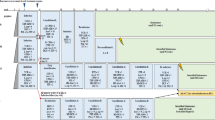
We present here the long-term results of three randomized clinical trials conducted on children with newly diagnosed acute lymphoblastic leukemia (ALL) between 1983 and 1998 by the Children Leukemia Cooperative Group (CLCG) from EORTC. In study 58831/32, the overall event-free survival (EFS) rates (± s.e.) at 6 and 10 years were 66% ± 1.8% and 65% ± 1.8%, respectively, and the risk of isolated central nervous system (CNS) relapse was 6% ± 1% and 7% ± 1%, respectively. In patients with a standard risk of relapse the omission of cyclophosphamide had no adverse effect on disease-free survival rates at 10 years (trial 58831). In medium- and high-risk patients the omission of radiotherapy did not increase the risk of CNS or systemic relapse (trial 58832). In study 58881 (1989–1998) the overall EFS rate at 8 years was 68.4% ± 1.2% and the risk of isolated CNS relapse was 4.2% ± 0.5%. In this trial which adressed three randomized questions, the following results were obtained: the combination of cytarabine at high doses with methotrexate at high doses during interval therapy did not improve prognosis. The addition of 6-mercaptopurine iv during maintenance increased the risk of late relapse. E. coli asparaginase was more toxic and has a higher efficacy than erwinia asparaginase. leukocyte counts >100 × 109/l, specific genetic abnormalities, a poor initial response to steroids or a high level of minimal residual disease at early time points were consistently associated with an adverse prognosis in the 58881 trial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rubie H, Benoit Y, Behar C, Lutz P, Maurus R, Philippe N, Plouvier E, Robert A, Sauveur E, Solbu G, Suciu S, Otten J . Treatment of standard risk (SR) acute lymphoblastic leukemia (ALL) with the BFM protocol with or without cyclophosphamide: preliminary results of a randomized trial (EORTC nr 58831). SIOP XX Meeting, Trondheim, Norway, 22–26 August 1988
Benoit Y, Boilletot A, Francotte N, Hoyoux C, Marguerite G, Philippe N, Souillet G, Thyss A, Solbu G, Suciu S, Otten J . Treatment of medium and high risk (M-HR) acute lymphoblastic leukemia (ALL) with or without radiotherapy (RXT): results of a randomized trial (EORTC nr 58832). SIOP XX Meeting, Trondheim, Norway, 22–26 August 1988
Millot F, Suciu S, Philippe N, Benoit Y, Nelken B, Uyttebroeck A, Mechinaud F, Vilmer E, Robert A, Boutard P, Marguerite G, Ferster A, Plouvier E, Rialland X, Behar C, Plantaz D, Dresse MF, Philippet P, Waterkeyn C, Otten J . Value of high dose Ara-C during the interval-therapy of a BFM like protocol in increased risk ALL and NHL stage III and IV patients: results of the EORTC 58881 trial Blood 1999 94: (Suppl. 1) (Abstr. 1700)
Millot F, Suciu S, Philippe N, Benoit Y, Mazingue F, Uyttebroeck A, Mechinaud F, Vilmer E, Robert A, Boutard P, Marguerite G, Ferster A, Plouvier E, Rialland X, Behar C, Plantaz D, Dresse MF, Philippet P, Waterkeyn C, Otten J for the Children Leukemia Cooperative Group (CLCG) of the European Organization for Research and Treatment of Cancer (EORTC) . Value of high-dose Ara-C during the interval-therapy of a BFM-based protocol in increased risk children with acute lymphoblastic leukemia and lymphoblastic lymphoma: results of the EORTC 58881 randomized phase III trial. (Submitted)
Van Der Werften Bosch J, Suciu S, Philippe N, Mazingue F, Vilmer E, Robert A, Mechinaud F, Marguerit G, Lutz P, Ferster A, Boutard P, Brock P, Muntzer M, Michel G, Plouvier E, Rialland X, Thyss C, Waterkeyn C, Solbu G, Otten J . The value of 6-MP i.v. during maintenance treatment in childhood acute lymphoblastic leukemia (ALL) and non Hodgkin lymphoma (NHL): results of the randomized phase III trial 58881 of EORTC Childhood Leukemia Cooperative Group (CLCG) Blood 1999 94: (Suppl. 1) (Abstr. 2791)
Otten J, Suciu S, Lutz P, Benoit Y, Robert A, Thyss A, Plouvier E, Ferster A, Mechinaud F, Mazingue F, Brock P, Vilmer E, Solbu G, Waterkeyn C, Philippe N, Van Camp B . The importance of L-asparaginase (A'ase) in the treatment of acute lymphoblastic leukemia (ALL) in children Blood 1996 88: (Suppl. 1) (Abstr. 2633)
Duval M, Suciu S, Ferster A, Benoit Y, Francotte N, Dresse MF, Uyttebroeck A, Plouvier E, Thyss A, Lutz P, Marguerite G, Behar C, Mazingue F, Boutard P, Millot F, Rialland X, Mechinaud F, Norton L, Robert A, Waterkeyn C, Vilmer E, Philippe N, Otten J . Comparative toxicity and efficacy of E. coli-asparaginase and Erwinia-asparaginase in childhood lymphoid malignancies: results of an EORTC-CLCG phase III randomized trial. (Submitted)
Langerman HJ, Henze G, Wulf M, Rhiem H . Abschatzung der tumorzellmasse bei der akuten lymphoblastischen leukamie im kindesalter: prognostische bedeuting und pratische Anwendung Klin Ped 1982 194: 209–213
Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddeman W, Niemeyer C, Henze G, Feldges A, Zint F, Kormhuber B, Riter J, Welte K, Gadner H, Riehm H . Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90 Blood 2000 95: 3310–3322
Mahmoud HH, Rivera GK, Hancock ML, Krance RA, Kun LE, Behm FG, Ribeiro RC, Sandlund JT, Crist WM, Pui CH . Low leucocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia New Engl J Med 1993 329: 314–319
Henze G, Buchmann S, Fengler R, Hartmann R . The BFM relapse studies in childhood ALL: concepts of two multicenter trials and results after 2½ years Hamatol Bluttransfus 1987 30: 147–155
Harris EK, Albert A . Survivorship Analysis for Clinical Studies M Dekker: New York 1991
Gardner MJ, Altman DG . Statistics with Confidence – Confidence Intervals and Statistical Guidelines British Medical Journal: London 1989
Thyss A, Suciu S, Bertrand Y, Mazingue F, Robert A, Vilmer E, Mechinaud F, Benoit Y, Brock P, Ferster A, Lutz P, Boutard P, Marguerite G, Plouvier E, Michel G, Plantaz D, Munze M, Rialland X, Chantraine JM, Norton L, Solbu G, Philippe N, Otten J . Systemic effect of intrathecal methotrexate during the initial phase of treatment of childhood acute lymphoblastic leukemia J Clin Oncol 1997 15: 1824–1830
Cavé H, Van Der Werff Ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E . Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia New Engl J Med 1998 339: 591–598
Ferster A, Benoit Y, Francotte N, Dresse MF, Uyttebroeck A, Plouvier E, Thyss A, Lutz P, Marguerite G, Behar C, Mazingue F, Boutard P, Millot F, Rialland X, Mechinaud F, Norton L, Robert A, Otten J, Vilmer E, Philippe N, Waterkeyn C, Suciu S . Treatment outcome in infant acute lymphoblastic leukemia Blood 2000 95: 2729–2731
Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, Odenwald E, Ritter J Mann G, Welte K, Gadner H, Riehm H . Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia. Results and conclusions of the multicenter trial ALL-BFM 86 Blood 1994 84: 3122–3133
Guidal C, Vilmer E, Grandchamp B, Cavé H . A semi-quantitative method using TcRγδ and IgH rearrangements for rapid detection of patients with high levels of minimal residual disease in acute lymphoblastic leukemia. (Submitted)
Loning L, Zimmermann M, Reiter A, Kaatsch P, Henze G, Riehm H, Schrappe M . Secondary neoplasms subsequent to Berlin–Frankfurt–Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy Blood 2000 95: 2770–2275
Pui CH, Mahmoud HH, Rivera GK, Hancock ML, Sandlund JT, Behm FG, Head DR, Relling MV, Ribeiro RC, Rubnitz JE, Kun LE, Evans WE . Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia Blood 1998 92: 411–415
Conter V, Shrappe M, Arico M, Reiter A, Rizzori C, Dordelmann M, Valsecchi MG, Zimmermann M, Ludwig WD, Basso G, Masera G, Riehm H . Role of cranial radiotherapy for chilhood T-cell acute lymphoblastic leukemia with high WBC count and good response to prednisone J Clin Oncol 1997 15: 2786–2791
Graham ML, Shuster JJ, Kamen BA, Land VJ, Borowitz MJ, Camitta B, Cheo DL, Harrison MP, Leventhal BG, Pinkel DP, Pullen DJ, Steuber P, Whitehead VM . Changes in red blood cell methotrexate pharmacology and their impact on outcome when cytarabine is infused with methotrexate in the treatment of acute lymphocytic leukemia in children: a Pediatric Oncology Group study Clin Cancer Res 1996 2: 331–337
Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE . Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia Blood 1999 93: 2817–2823
Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ . Comparative pharmacokinetics studies of three asparaginase preparations J Clin Oncol 1993 11: 1780–1786
Boos J, Weber G, Ahlke E, Schulze WP, Nowak GO, Wurthwein G, Boos J, Werber G, Ahlke E, Schulze-Westhoff P, Nowak-Gottl U, Wurthwein G, Verspohl EJ, Ritter J, Jurgens H . Monitoring of asparaginase activity and asparaginase levels in children on different asparaginase preparations Eur J Cancer 1996 32A: 1544–1550
Acknowledgements
This publication was supported by grant number 5U10-CA11488–13 through 5U10-CA11488–30 from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vilmer, E., Suciu, S., Ferster, A. et al. Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Leukemia 14, 2257–2266 (2000). https://doi.org/10.1038/sj.leu.2401960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401960
Keywords
This article is cited by
-
Population Pharmacokinetic Model of Methotrexate in Brazilian Pediatric Patients with Acute Lymphoblastic Leukemia
Pharmaceutical Research (2023)
-
Safety of intrathecal route: focus to methylprednisolone acetate (Depo-Medrol) use
European Spine Journal (2019)
-
Differential impact of drugs on the outcome of ETV6-RUNX1 positive childhood B-cell precursor acute lymphoblastic leukaemia: results of the EORTC CLG 58881 and 58951 trials
Leukemia (2018)
-
Asparaginase-associated pancreatitis: a study on phenotype and genotype in the NOPHO ALL2008 protocol
Leukemia (2017)
-
The evolution of clinical trials for infant acute lymphoblastic leukemia
Blood Cancer Journal (2014)