Abstract
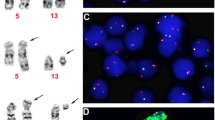
Since deletion of chromosome 13q is a clinically relevant feature in multiple myeloma (MM), we analyzed bone marrow plasma cells from 29 patients with monoclonal gammopathy of undetermined significance (MGUS) to investigate the chromosome 13 status in MGUS. Studies were performed by interphase fluorescence in situ hybridization (FISH) with a panel of 13q14-specific probes (RB1, D13S319, D13S25, D13S31). Plasma cells with a deletion of at least one of the 13q14 loci were detected in 13 patients (44.8%) with MGUS. In five patients (17.2%), deletions of all four 13q14-specific probes were observed, and the additional deletion of a 13q telomeric region (D13S327) suggested loss of the entire 13q arm or monosomy 13. Loss of 13q14 was observed to be monoallelic and to occur in 11.0 to 35.0% of plasma cells (cut-off levels for a deletion <10% with all probes). Nine of 17 patients (52.9%) with MM progressing from a pre-existing MGUS had evidence for a deletion of 13q14 as determined by FISH with the RB1 probe. These results suggest that deletion of 13q14 is an early event in the development of monoclonal gammopathies, but its role for the eventual progression to MM remains to be determined prospectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hallek M, Leif-Bergsagel P, Anderson KC . Multiple myeloma: increasing evidence for a multistep transformation process Blood 1998 91: 3–21
Zandecki M, Lai J-L, Facon T . Almost all patients with multiple myeloma are cytogenetically abnormal Br J Haematol 1996 94: 217–227
Tricot G, Barlogie B, Jagannath S, Bracy D, Mattox S, Vesole DH, Naucke S, Sawyer JR . Poor prognosis in multiple myeloma is associated only with partial or complete deletions of chromosome 13 or abnormalities involving 11q and not with other karyotype abnormalities Blood 1995 86: 4250–4256
Seong C, Delasalle K, Hayes K, Weber D, Dimopoulos M, Swantkowski J, Huh Y, Glassman A, Champlin R, Alexanian R . Prognostic value of cytogenetics in multiple myeloma Br J Haematol 1998 101: 189–194
Perez-Simon SJ, Garcia SR, Tabernero MD, Almeida J, Gonzalez M, Fernandez CJ, Moro MJ, Hernandez JM, San Miguel J, Orfao A . Prognostic value of numerical chromosome aberrations in multiple myeloma: a FISH analysis of 15 different chromosomes Blood 1998 91: 3366–3371
Zojer N, Königsberg R, Ackermann J, Fritz E, Dallinger S, Krömer E, Kaufmann H, Riedl L, Gisslinger H, Schreiber S, Heinz R, Ludwig H, Huber H, Drach J . In multiple myeloma, deletion of 13q14 remains an independent adverse prognostic parameter despite its frequent detection by interphase FISH Blood 2000 95: 1925–1930
Calasanz MJ, Cigudosa JC, Odero MD, Ferreira C, Ardanaz MT, Fraile A, Carrasco JL, Sole F, Cuesta B, Gullon A . Cytogenetic analysis of 280 patients with multiple myeloma and related disorders: primary breakpoints and clinical correlations Genes Chromosomes Cancer 1997 18: 84–93
Drach J, Angerler J, Schuster J, Rothermundt C, Thalhammer R, Haas OA, Jäger U, Fiegl M, Geissler K, Ludwig H, Huber H . Interphase fluorescence in situ hybridization identifies chromosomal abnormalities in plasma cells from patients with monoclonal gammopathy of undetermined significance Blood 1995 86: 3915–3921
Zandecki M, Lai JL, Genevieve F, Bernardi F, Volle-Remy H, Blanchet O, Francois M, Cosson A, Bauters F, Facon T . Several cytogenetic subclones may be identified within plasma cells from patients with monoclonal gammopathy of undetermined significance, both at diagnosis and during the indolent course of this condition Blood 1997 90: 3682–3690
Ahmann GJ, Jalal SM, Juneau AL, Christensen ER, Hanson CA, Dewald GW, Greipp PR . A novel three-color, clone-specific fluorescence in situ hybridization procedure for monoclonal gammopathies Cancer Genet Cytogenet 1998 101: 7–11
Shaughnessy J, Tian E, Bell T, Xiao Y, Spoon D, Butch A, Drach J, Sawyer J, Barlogie B . Molecular cytogenetic analysis of chromosome 13q14, site of a putative tumor suppressor gene in multiple myeloma (TSG-MM) Blood 1998 92: (Suppl. 1) 259a
Kyle RA, Lust JA . Monoclonal gammopathies of undetermined significance Sem Hematol 1989 26: 176–200
Greipp PR . Monoclonal gammopathies: new approaches to clinical problems in diagnosis and prognosis Blood Rev 1989 3: 222–236
Kalachikov S, Migliazza A, Cayanis E, Fracchiolla NS, Bonaldo MF, Lawton L, Jelenc P, Ye X, Qu X, Chien M, Hauptschein R, Gaidano G, Vitolo U, Saglio G, Resegotti L, Brodjansky V, Yankovsky N, Zhang P, Soares MB, Russo J, Edelman IS, Efstratiadis A, Dalla-Favera R, Fischer SG . Cloning and gene mapping of the chromosome 13q14 region deleted in chronic lymphocytic leumekia Genomics 1997 42: 369–377
Drach J, Schuster J, Nowotny H, Angerler J, Rosenthal F, Fiegl M, Rothermundt C, Gsur A, Jäger U, Heinz R, Lechner K, Ludwig H, Huber H . Multiple myeloma: high incidence of chromosomal aneuploidy as detected by interphase fluorescence in situ hybridization Cancer Res 1995 55: 3854–3859
Ackermann J, Meidlinger P, Zojer N, Gisslinger H, Ludwig H, Huber H, Drach J . Absence of p53 deletions in bone marrow plasma cells of patients with monoclonal gammopathy of undetermined significance Br J Haematol 1998 103: 1161–1163
Dao DD, Sawyer JR, Epstein J, Hoover RG, Barlogie B, Tricot G . Deletion of the retinoblastoma gene in multiple myeloma Leukemia 1994 8: 1280–1284
Chang H, Bouman D, Boerkoel CF, Stewart AK, Squire JA . Frequent monoallelic loss of D13S319 in multiple myeloma patients shown by interphase fluorescence in situ hybridization Leukemia 1999 13: 105–109
Avet-Loiseau H, Li JY, Morineau N, Facon T, Brigaudeau C, Harousseau JL, Grosbois B, Bataille R . Monosomy 13 is associated with the transition of monoclonal gammopathy of undetermined significance to multiple myeloma Blood 1999 94: 2583–2589
Fonseca R, Aguayo P, Ahmann GJ, Jalal SM, Rajkumar SV, Kyle RA, Gertz MA, Dewald GW, Dispenzieri A, Lust JA, Lacy MQ, Witzig TE, Greipp PR . Translocations at 14q32 are common in patients with the monoclonal gammopathy of undetermined significance (MGUS) and involve several partner chromosomes Blood 1999 94: (Suppl. 1) 663a
Kyle RA . ‘Benign’ monoclonal gammopathy – after 20 to 35 years of follow-up Mayo Clin Proc 1993 68: 26–36
Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL, Vives-Corrons JL, Montserrat E . Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance Br J Haematol 1992 81: 391–394
Baldini L, Guffanti A, Cesana BM, Colombi M, Chiorboli O, Damilano I, Maiolo AT . Role of different hematologic variables in defining the risk of malignant transformation in monoclonal gammopathy Blood 1996 87: 912–918
Bataille R, Chappard D, Basle MF . Quantifiable excess of bone resorption in monoclonal gammopathy is an early symptom of malignancy: a prospective study of 87 bone biopsies Blood 1996 87: 4762–4769
Gernone A, Dammacco F . Molecular alterations of IL-6R, lck and c-myc genes in transforming monoclonal gammopathies of undetermined significance Br J Haematol 1996 93: 623–631
Acknowledgements
This work was supported by the Austrian ‘Fonds zur Förderung der wissenschaftlichen Forschung’ (Grant No. P12432-MED) and the ICP-Programme (Molecular Medicine) of the Austrian Ministry for Research and Transport.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Königsberg, R., Ackermann, J., Kaufmann, H. et al. Deletions of chromosome 13q in monoclonal gammopathy of undetermined significance. Leukemia 14, 1975–1979 (2000). https://doi.org/10.1038/sj.leu.2401909
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401909
Keywords
This article is cited by
-
Both IGH translocations and chromosome 13q deletions are early events in monoclonal gammopathy of undetermined significance and do not evolve during transition to multiple myeloma
Leukemia (2004)
-
Multiple myeloma: evolving genetic events and host interactions
Nature Reviews Cancer (2002)
-
Deletion analysis of chromosome 13q14.3 and characterisation of an alternative splice form of LEU1 in B cell chronic lymphocytic leukemia
Leukemia (2002)
-
Integrating cytogenetics and gene expression profiling in the molecular analysis of Multiple Myeloma
International Journal of Hematology (2002)