Abstract
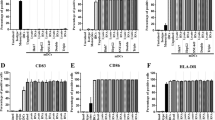
The recent clinical trial in lymphoma using tumor antigen-loaded DCs (Hsu et al, Nature Med 1996; 2: 52) demonstrates the efficiency of the use of professional antigen presenting cells (APCs) for taking up, processing and presenting tumor protein in a vaccine strategy in cancer. However, the production of large quantities of clinical grade APCs remains to be resolved. Here, we describe that both dendritic cells (DCs) and macrophages (MØs) can be efficiently differentiated in large numbers from lymphoma patients in spite of their disease and previous therapy. These cells were produced using the VAC and MAK cell processors according to standard operating procedures. DCs and MØs were differentiated from circulating monocytes in gas permeable hydrophobic bags, with 2% autologous serum and in the presence of GM-CSF and IL-13 or GM-CSF alone, respectively. DCs and MØs were then purified by counter flow centrifugation. Phenotypic, morphological and functional analysis showed that cells differentiated from patients with lymphoma present quite similar features to DCs and MØs produced from monocytes of healthy donors. Moreover, we show that MØs, when combined with CD20 antibody (Rituximab), can efficiently engulf tumor cells and propose that a such combination could be used for initiating a clinical trial in lymphoma. Thus, the possibility of producing functional DC and MØs in large amounts in conditions compatible with therapeutic application will allow the development of new immune strategies to eradicate lymphoma. Leukemia (2000) 14, 1667–1677.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Horning SJ . Natural history of and therapy for the indolent non-Hodgkin’s lymphomas Semin Oncol 1993 20: 75–88
Jacob MC, Piccini MP, Bonnefoix T, Sotto MF, Couderc P, Bensa JC, Sotto JJ . T lymphocytes from invaded lymph nodes in patients with B-cell-derived non-Hodgkin’s lymphoma: reactivity toward the malignant clone Blood 1990 75: 1154–1162
Schwartzentruber DJ, Steler-Stevenson M, Rosenberg SA, Topalian SL . Tumor-infiltrating lymphocytes from select B-cell lymphomas secrete granulocyte–macrophage colony-stimulating factor and tumor necrosis factor-α in response to autologous tumor stimulation Blood 1993 82: 1204–1211
Chaperot L, Delfeau-Larue M-H, Jacob M-C, Molens J-P, Roussel B, Agrawal S, Farcet J-P, Gressin R, Sotto J-J, Bensa J-C, Plumas J . Differentiation of antitumor-specific cytotoxic T lymphocytes from autologous tumor infiltrating lymphocytes in non-Hodgkin’s lymphomas Exp Hematol 1999 27: 1185–1193
Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM . Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro Blood 1997 89: 3806–3816
Plumas J, Jacob MC, Chaperot L, Molens JP, Sotto JJ, Bensa JC . Tumor B cells from non-Hodgkin’s lymphoma are resistant to CD95 (Fas/Apo-1)-mediated apoptosis Blood 1998 91: 2875–2885
Nelson EL, Li X, Hsu FJ, Kwak LW, Levy R, Clayberger C, Krensky AM . Tumor specific cytotoxic T-lymphocyte response after idiotype vaccination for B-cell non-Hodgkin’s lymphoma Blood 1996 88: 580–589
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R . Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells Nature Med 1996 2: 52–58
Nestle FO, Alijagic S, Gillet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D . Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells Nature Med 1998 4: 328–332
Höltl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M . CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal cell cancer Lancet 1998 352: 1358
Brossart P, Bevan MJ . Presentation of exogenous protein antigens on major histocompatability complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines Blood 1997 90: 1594–1599
Svensson M, Stockinger B, Wick MJ . Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells J Immunol 1997 158: 4229–4236
Regnault A, Lankar D, Lacabanne V, Rodriguez A, Héry C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S . Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization J Exp Med 1999 189: 371–380
Harding CV, Song R . Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules J Immunol 1994 153: 4925–4933
Kovacsovics-Bankowski M, Rock KL . A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules Science 1995 267: 243–246
Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV . Phagocytic processing of bacterial antigens for class I MHC presentation to T cells Nature 1993 361: 359–362
Toujas L, Delcros JG, Diez E, Gervois N, Semana G, Corradin G, Jotereau F . Human monocyte-derived macrophages and dendritic cells are comparably effective in vitro in presenting HLA class I-restricted exogenous peptides Immunology 1997 91: 635–642
Andreesen R, Hennemann B, Krause SW . Adoptive immunotherapy of cancer using monocyte-derived macrophages: rationale, current status, and perspectives J Leuk Biol 1998 64: 419–426
Bartholeyns J, Romet Lemonne JL, Chokri M, Lopez M . Immune therapy with macrophages: present status and critical requirements for implementation Immunobiology 1996 195: 550–562
Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl HG, Leser HG, Engler H, Löhr GW . Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy Cancer Res 1990 50: 7450–7456
Eymard JC, Lopez M, Cattan A, Bouche O, Adjizian JC, Bernard J . Phase I/II trial of autologous activated macrophages in advanced colorectal cancer Eur J Cancer 1996 32a: 1905–1911
Lopez M, Fechtenbaum J, David B, Martinache C, Chokri M, Canepa S, De Gramont A, Louvet C, Gorin I, Mortel O et al. Adoptive immunotherapy with activated macrophages grown in vitro from blood monocytes in cancer patients: a pilot study J Immunother 1992 11: 209–217
Ely P, Wallace PK, Givan AL, Graziano RF, Guyre PM, Fanger MW . Bispecific-armed, interferon γ-primed macrophage-mediated phagocytosis of malignant non-Hodgkin’s lymphoma Blood 1996 87: 3813–3821
Chokri M, Girard A, Borrelly MC, Oleron C, Romet-Lemone JL, Bartholeyns J . Adoptive immunotherapy with bispecific antibodies: targeting through macrophages Res Immunol 1992 143: 95–99
Desmedt M, Rootiers P, Dooms H, Fiers W, Grooten J . Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses J Immunol 1998 160: 5300–5308
Wijburg OLC, van den Dobbelsteen GPJM, Vadolas J, Sanders A, Strugnell RA, van Rooijen N . The role of macrophages in the induction and regulation of immunity elicited by exogenous antigens Eur J Immunol 1998 28: 479–487
Hart DNJ . Dendritic cells: unique leukocyte populations which control the primary immune response Blood 1997 90: 3245–3287
Hsu FJ, Caspar CB, Czerwinski D, Kwak LW, Liles TM, Syrengelas A, TaidiLaskowski B, Levy R . Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma – long-term results of a clinical trial Blood 1997 89: 3129–3135
Murphy G, Tjoa B, Ragde H, Kenny G, Boyton A . Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen Prostate 1996 29: 371–380
Chokri M, Lopez M, Oleron C, Girard A, Martinache C, Canepa S, Siffert JC, Bartholeyns J . Production of human macrophages with potent antitumor properties (MAK) by culture of monocytes in the presence of GM-CSF and 1, 25-dihydroxy vitamin D3 Anticancer Res 1992 12: 2257–2261
Goxe B, Latour N, Bartoleyns J, Romet Lemonne JL, Chokri M . Monocyte-derived dendritic cells: development of a cellular processor for clinical applications Res Immunol 1998 149: 643–646
Boyer A, Andreu G, Romet Lemoine JL, Fridman WH, Teillaud JL . Generation of phagocytic Mak and Mac-DC for therapeutic use: characterization and in vitro functional properties Exp Hematol 1999 27: 751–761
Chaperot L, Plumas J, Jacob M-C, Bost F, Molens J-P, Sotto J-J, Bensa J-C . Functional expression of CD80 and CD86 allows immunogenicity of malignant B cells from non-Hodgkin’s lymphomas Exp Hematol 1999 27: 479–488
Reis e Sousa C, Germain RN . Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis J Exp Med 1995 182: 841–851
Cannon GJ, Swanson JA . The macrophage capacity for phagocytosis J Cell Sci 1992 101: 907–913
Inaba K, Inaba M, Naito M, Steinman RM . Dendritic cells progenitors phagocytose particulates, including bacillus Calmette–Guerin organisms, and sensitize mice to mycobacterial antigens in vivo J Exp Med 1993 178: 479–488
Reis e Sousa C, Stahl PD, Austyn JM . Phagocytosis of antigens by langerhans cells in vitro J Exp Med 1993 178: 509–519
Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA . Processing of engulfed apoptotic bodies yields T cell epitopes J Immunol 1997 159: 5391–5399
Albert ML, Sauter B, Bhardwaj N . Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs Nature 1998 392: 86–89
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM . Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF J Clin Invest 1998 101: 890–898
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo Lopez A, Levy R . Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma Blood 1994 84: 2457–2466
Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R . Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors New Engl J Med 1992 327: 1209–1215
Böhm W, Schirmbeck R, Elbe A, Melber K, Diminky D, Kraal G, Van Rooijen N, Barenholz Y, Reimann J . Exogenous hepatitis B surface antigen particles processed by dendritic cells or macrophages prime murine MHC class I restricted cytotoxic T lymphocytes in vivo J Immunol 1995 155: 3313–3321
Trinchieri G, Perussia B . Immune interferon: a pleiotropic lymphokine with multiple effects Immunol Today 1985 6: 961–971
Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E . Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors J Exp Med 1997 186: 1177–1182
Nair SK, Snyder D, Rouse B, Gilboa E . Regression of tumors in mice vaccinated with professional antigen-presenting cells pulsed with tumor extracts Int J Cancer 1997 70: 706–715
Fields RC, Shimizu K, Mulé JJ . Murine dendritic cells pulsed whith whole tumor lysates mediate potent antitumor immune response in vitro and in vivo Proc Natl Acad Sci USA 1998 95: 9482–9487
Dyall R, Vasovic LV, Clynes RA, Nikolic-Zugic J . Cellular requirements for the monoclonal antibody-mediated eradication of an established solid tumor Eur J Immunol 1999 29: 30–37
Fanger NA, Wardwell K, Shen L, Tedder TF, Guyre PM . Type I (CD64) and type II (CD32) Fcgamma receptor-mediated phagocytosis by human blood dendritic cells J Immunol 1996 157: 541–548
Acknowledgements
We are grateful to the staff of the blood center immunological and cell therapy departments for their collaboration. This work was supported by grant No. 9336 from ‘Association pour la Recherche sur le cancer’ and by a grant from ‘Ligue Contre le Cancer – Comité de la Savoie’.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaperot, L., Chokri, M., Jacob, MC. et al. Differentiation of antigen-presenting cells (dendritic cells and macrophages) for therapeutic application in patients with lymphoma. Leukemia 14, 1667–1677 (2000). https://doi.org/10.1038/sj.leu.2401888
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401888