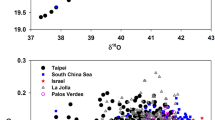
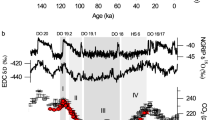
Abstract
Oxygen has three naturally occurring isotopes, of mass numbers 16, 17 and 18. Their ratio in atmospheric O2 depends primarily on the isotopic composition of photosynthetically produced O2 from terrestrial and aquatic plants1,2,3, and on isotopic fractionation due to respiration4. These processes fractionate isotopes in a mass-dependent way, such that 17O enrichment would be approximately half of the 18O enrichment relative to 16O. But some photochemical reactions in the stratosphere give rise to a mass-independent isotope fractionation, producing approximately equal 17O and 18O enrichments in stratospheric ozone5 and carbon dioxide6,7, and consequently driving an atmospheric O2 isotope anomaly. Here we present an experimentally based estimate of the size of the 17O/16O anomaly in tropospheric O2, and argue that it largely reflects the influences of biospheric cycling and stratospheric photochemical processes. We propose that because the biosphere removes the isotopically anomalous stratosphere-derived O2 by respiration, and replaces it with isotopically ‘normal’ oxygen by photosynthesis, the magnitude of the tropospheric 17O anomaly can be used as a tracer of global biosphere production. We use measurements of the triple-isotope composition of O2 trapped in bubbles in polar ice to estimate global biosphere productivity at various times over the past 82,000 years. In a second application, we use the isotopic signature of oxygen dissolved in aquatic systems to estimate gross primary production on broad time and space scales.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Guy, R. D., Fogel, M. L. & Berry, J. A. Photosynthetic fractionation of stable isotopes. Plant. Physiol. 101, 37–47 (1993).
Dongmann, G. The contribution of land photosynthesis to the stationary enrichment of the 18O in the atmosphere. Radiat. Environ. Biophys. 11, 219–255 (1974).
Farquhar, G. D. et al. Vegetation effects on the isotope composition of oxygen in atmospheric CO2. Nature 363, 439–443 (1993).
Lane, G. & Dole, M. Fractionation of oxygen isotopes during respiration. Science 123, 574–576 (1956).
Schueler, B., Morton, J. & Mauersberger, K. Measurement of isotopic abundances in collected stratospheric ozone samples. Geophys. Res. Lett. 17, 1295–1298 (1990).
Thiemens, M. H., Jackson, T., Zipf, E. C., Erdman, P. W. & van Egmond, C. Carbon dioxide and oxygen isotope anomalies in the mesosphere and stratosphere. Science 270, 969–972 (1995).
Thiemens, M. H., Jackson, T. L. & Brenninkmeijer, C. A. M. Observation of a mass independent oxygen isotopic composition in terrestrial stratospheric CO2, the link to ozone chemistry, and the possible occurrence in the Martian atmosphere. Geophys. Res. Lett. 22, 225–257 (1995).
Thiemens, M. H. Mass-independent isotope effects in planetary atmospheres and the early solar system. Science 283, 341–345 (1999).
Li, W. J. & Meijer, H. A. J. The use of electrolysis for accurate δ17O and δ18O isotope measurements in water. Isotopes Environ. Health Studies 34, 349–369 (1998).
Bender, M., Sowers, T. & Labeyrie, L. The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core. Global Biogeochem. Cycles 8, 363–376 (1994).
Thiemens, M. H., Jackson, T., Maurersberger, K., Schueler, B. & Morton, J. Oxygen isotope fractionation in stratospheric CO2. Geophys. Res. Lett. 18, 669–672 (1991).
Thiemens, M. H. & Jackson, T. Pressure dependency for heavy isotope enhancement in ozone formation. Geophys. Res. Lett. 17, 717–719 (1990).
Mauersberger, K., Erbacher, B., Kranowsky, D., Gunther, J. & Nickel, R. Ozone isotope enrichment: Isotopomer-specific rate coefficients. Science 283, 370–372 (1999).
Yung, Y. L., Lee, A. Y. T., Irion, F. W., DeMore, W. B. & Wen, J. Carbon dioxide in the atmosphere: Isotopic exchange with ozone and its use as a tracer in the middle atmosphere. J. Geophys. Res. 102, 10857–10866 (1997).
Wen, J. & Thiemens, M. H. First multi-isotope study of the O(1D) + CO2exchange and stratospheric consequences. J. Geophys. Res. 98, 12801–12808 (1993).
Francey, R. J. & Tans, P. P. Latitudinal variations in oxygen-18 of atmospheric CO2. Nature 327, 495–497 (1987).
Dole, M. & Jenks, G. Isotopic composition of photosynthetic oxygen. Science 100, 409 (1944).
Minschwaner, K., Salawitch, R. J. & McElroy, M. B. Absorption of solar radiation by O2: Implications for O3and lifetimes of N2O, CFCl3, and CF2Cl2. J. Geophys. Res. 98, 10543–10561 (1993).
Boering, K. A. et al. Stratospheric mean ages and transport rates from observations of carbon dioxide and nitrous oxide. Science 274, 1340–1343 (1996).
Holton, J. R. On the global exchange of mass between the stratosphere and the troposphere. J. Atmos. Sci. 47, 392–395 (1990).
Appenzeller, C., Holton, J. R. & Rosenlof, K. H. Seasonal variation of mass transport across the tropopause. J. Geophys. Res. 101, 15071–15078 (1996).
Crutzen, P. J. & Bruhl, C. Amodel study of atmospheric temperatures and the concentrations of ozone, hydroxyl, and some other photochemically active gases during the glacial, the pre-industrial Holocene and the Present. Geophys. Res. Lett. 20, 1047–1050 (1993).
Martinerie, P., Brasseur, G. P. & Granier, C. The chemical composition of ancient atmospheres: A model study constrained by ice core data. J. Geophys. Res. 100, 14291–14304 (1995).
Rind, D. & Lacis, A. The role of the stratosphere in climate change. Surv. Geophys. 14, 133–165 (1993).
Meyer, M. K. Net Primary Productivity Estimates for the Last 18,000 years Evaluated from Simulations by a Global Climate Model.Thesis, Univ. Wisconsin(1988).
Clark, J. F. et al. in Air-Water Gas Transfer (eds Jaehne, B. & Monahan, E. C.) 785–800 (Aeon, Hanau, (1995).
Sowers, T., Bender, M. & Raynaud, D. Elemental and isotopic composition of occluded O2and N2in polar ice. J. Geophys. Res. 94, 5137–5150 (1989).
Brenninkmeijer, C. A. M., Lowe, D. C., Manning, M. R., Sparks, R. J. & van Velthoven, P. F. J. The 13C, 14C and 18O isotopic composition of CO, CH4, and CO2in the higher southern latitudes lower stratosphere. J. Geophys. Res. 100, 26163–26172 (1995).
Barnola, J. M., Pimienta, P., Raynaud, D. & Korotkevich, Y. S. CO2–climate relationship as deduced from the Vostok ice core—A reexamination based on new measurements and on a reevaluation of the air dating. Tellus B 43, 83–90 (1991).
Acknowledgements
We appreciate the help of Y. Yacobi, and thank J. Orchardo and Y. Sagi for help with sample preparation. We thank the USA-Israel BSF, The Israel Science Foundation and the Moshe-Shilo Minerva Center for support; we also thank the Office of Polar Programs of the NSF, and the National Institute of Global Environmental Change, Department of Energy for their support of the ice-core study. M.H.T. thanks the NSF for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luz, B., Barkan, E., Bender, M. et al. Triple-isotope composition of atmospheric oxygen as a tracer of biosphere productivity. Nature 400, 547–550 (1999). https://doi.org/10.1038/22987
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22987
This article is cited by
-
New constraints of terrestrial and oceanic global gross primary productions from the triple oxygen isotopic composition of atmospheric CO2 and O2
Scientific Reports (2023)
-
Sulfate triple-oxygen-isotope evidence confirming oceanic oxygenation 570 million years ago
Nature Communications (2023)
-
Managing argon interference during measurements of 18O/16O ratios in O2 by continuous-flow isotope ratio mass spectrometry
Analytical and Bioanalytical Chemistry (2022)
-
Exceptionally high biosphere productivity at the beginning of Marine Isotopic Stage 11
Nature Communications (2020)
-
Triple oxygen isotope evidence for limited mid-Proterozoic primary productivity
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.