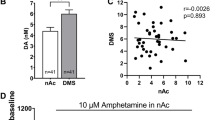
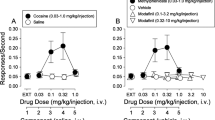
Abstract
IN attempts to work out possible mechanisms of action of the hallucinogenic drugs, it would be helpful to know which chemical groups modify their activity. In the methoxylated amphetamines, some methoxy groups may act as electrostatic binding sites and some may effect the shape and energy distribution of the π cloud. It may also be important to know whether the amino group is necessary for activity. It has been shown that N,N-dimethylmescaline is a less potent compound than mescaline1,2. We have now tested further N,N-dimethyl derivatives of hallucinogenic amphetamines using the Bovet-Gatti profiles on the Sidman avoidance schedule3,4 which we have found to discriminate between hallucinogenie drugs very well. 4-Methoxyamphetamine is a very potent disrupting drug in rats and produces a typical “hallucinogenic” profile at 3 mg/kg (equivalent to mescaline at 25 mg/kg). The N,N-dimethyl derivative was totally inactive at 25 mg/kg. 2,5-Dimethoxy-4-methyl amphetamine (DOM) is the most potent human hallucinogenic amphetamine known (active in rats at 2.5 mg/kg). Its N,N-dimethyl derivative was also quite inactive at 20 mg/kg. These results suggest that, if these compounds bond, they do so using their N atoms, in which case our hypothesis5 would predict that the 3-compound (not yet tested) would be an active hallucinogen. The relative activity of methoxylated amphetamines seems to correlate more with their quantum chemical properties, such as their Hückel molecular orbital (HMO) energies and degree of native fluorescence6, than with their steric properties. That is to say, the steric requirements seem to be an unhindered N and an unhindered ortho or para methoxy group, and the energetic requirement is a high HMO energy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Smythies, J. R., and Sykes, E. A., Psychopharmacologia, 9, 434 (1966).
Smythies, J. R., Bradley, R. J., and Johnston, V. S., Brit. J. Psychiat., 115, 55 (1968).
Smythies, J. R., Bradley, R. J., and Johnston, V. S., Nature, 216, 196 (1967).
Smythies, J. R., Bradley, R. J., and Johnston, V. S., Nature, 220, 800 (1968).
Smythies, J. R., Benington, F., and Morin, R. D., NRP Bulletin, 8, 117 (1970).
Snyder, S. H., and Richelson, E., Proc. US Nat. Acad. Sci., 60, 206 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SMYTHIES, J., BEATON, J., BENINGTON, F. et al. Behavioural Effects of some Derivatives of Amphetamine and LSD and their Significance. Nature 226, 644–645 (1970). https://doi.org/10.1038/226644a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/226644a0
This article is cited by
-
Asymmetric amination of 4-methoxyphenylacetone and its related compounds with microorganisms
Applied Microbiology and Biotechnology (1990)
-
Blockage of LSD binding at its high affinity site on synaptosomal membranes by 1-methyl-1,2,5,6-tetrahydropyridine-N,N-diethyl-carboxamide
Experientia (1975)
-
Comparative Effects of Stereoisomers of Hallucinogenic Amphetamines
Nature New Biology (1973)
-
Some neurochemical and neuropharmacological studies on the interactions between mescaline and 1-methyl-1,2,5,6-tetrahydropyridine-3-(N,N-diethylcarboxamide) (THPC)
Psychopharmacologia (1972)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.