Abstract
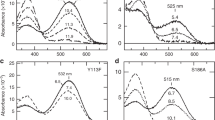
AN as yet unexplained aspect of visual pigment chemistry is the large red-shift in the absorption maximum of retinaldehyde, when it is bound to opsin in the visual pigment rhodopsin. The lipoprotein opsin does not absorb in the visible and near ultraviolet, 11-cis retinaldehyde has a λmax of 376 nm (in ethanol), while cattle rhodopsin has a λmax of 498 nm. In the latter substance retinaldehyde has been shown to be bound to an amino group in opsin by a Schiff base link1. Because a retinylidene Schiff base in neutral aqueous environment has a λmax about 20 nm lower than the free aldehyde, the discrepancy with the λmax of rhodopsin is about 140 nm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morton, R. A., and Pitt, G. A. J., Biochem. J., 59, 128 (1955).
Wald, G., and Brown, P. K., J. Gen. Physiol., 35, 797 (1952).
Peskin, J. C., and Love, B. B., Biochim. Biophys. Acta, 78, 753 (1963).
Mizuno, K., Ozawa, K., and Kuno, Y., Exp. Eye Res., 5, 276 (1966).
Mizuno, K., Kuno, U., and Ozawa, K., Jap. J. Ophthalmol., 10, 85 (1966).
Galindo, I. G., Bull. Math. Biophys., 29, 677 (1967).
Blatz, P. E., J. Gen. Physiol., 48, 753 (1965).
Blatz, P. E., and Pippert, D. L., J. Amer. Chem. Soc., 90, 1296 (1968).
Blatz, P. E., Pippert, D. L., and Balasubramaniyan, V., Photochem. Photobiol., 8, 309 (1968).
Rosenberg, B., and Krigas, T. P., Photochem. Photobiol., 6, 769 (1967).
Erickson, J. O., and Blatz, P. E., Vision Res., 8, 1367 (1968).
Bownds, D., and Wald, G., Nature, 205, 254 (1965).
Bownds, D., Nature, 216, 1178 (1967).
Akhtar, M., Blosse, P. T., and Dewhurst, P. B., Life Sci., 4, 1221 (1965).
Akhtar, M., Blosse, P. T., and Dewhurst, P. B., Chem. Commun., 631 (1967).
Krinsky, N. I., AMA Arch. Ophthalmol., 60, 688 (1958).
Bonting, S. L., and Bangham, A. D., Exp. Eye Res., 6, 400 (1967).
Bonting, S. L., and Bangham, A. D., in Biochemistry of the Eye (edit. by Dardenne, M. U., and Nordman, J.), 493 (S. Karger, Basel, New York, 1968).
Poincelot, R. P., Millar, P. G., Kimbel, R. L., and Abrahamson, E. W., Nature, 221, 256 (1969).
Adams, R. G., in Proc. Int. Symp. Biochem. of the Eye, Nijmegen, The Netherlands, June 1968; Exp. Eye Res., 8, 2 (1969).
Daemen, F. J. M., Chem. Phys. Lipids, 1, 476 (1967).
Crescitelli, F., Mommaerts, W. F. H. M., and Shaw, T. I., Proc. US Nat. Acad. Sci., 56, 1729 (1966).
Takezaki, M., and Kito, Y., Nature, 215, 1197 (1967).
Wald, G., and Hubbard, R., in The Enzymes (edit. by Boyer, P. D., Lardy, H., and Myrbäck, K.), 3, 369 (Academic Press, New York and London, 1960).
Takagi, M., Biochim. Biophys. Acta, 66, 328 (1963).
Sekoguti, Y., Takagi, M., and Kot, Y., Ann. Rep. Sci. Works, Fac. Sci., Osaka Univ., 12, 67 (1964).
Hubbard, R., Bownds, D., and Yoshizawa, T., Cold Spring Harbor Symp. Quant. Biol., 30, 301 (1965).
Kito, Y., and Takezaki, M., Nature, 211, 197 (1966).
Jencks, W. P., Prog. Phys. Org. Chem., 2, 63 (1964).
Wald, G., Durell, J., and St George, R. C. C., Science, 111, 179 (1950).
Bonting, S. L., Current Topics Bioenerg., 3, 351 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DAEMEN, F., BONTING, S. Internal Protonation in Retinylidene Phosphatidylethanolamine and the Red-shift in Rhodopsin. Nature 222, 879–881 (1969). https://doi.org/10.1038/222879a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/222879a0
This article is cited by
-
Linkage of Retinal to Opsin
Nature New Biology (1971)
-
Is Retinal-Phosphatidyl Ethanolamine the Chromophore of Rhodopsin?
Nature (1970)
-
Linkage of Retinal to Opsin and Absence of Phospholipids in Purified Frog Visual Pigment500
Nature (1970)
-
Phospholipid—Retinaldehyde Complex absorbing at 500 nm
Nature (1970)
-
Charge Delocalization in the Prosthetic Group of Visual Pigments
Nature (1969)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.