Abstract
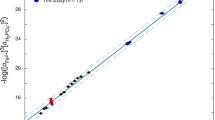
MOST of the elements in solution in sea water are present in smaller amounts than would be expected on the basis of solubility equilibria. The concentrations of many of these elements are controlled by other processes such as ion exchange, adsorption and biological removal. There are, nevertheless, several elements for which solubility may be an important factor affecting abundance and distribution in parts of the ocean. These generalizations are based on comparisons1,2 of the observed concentrations of elements in sea water with those which would be expected from a consideration of their solubility. Even when reliable abundance data are available, however, exact comparisons are precluded by the lack of accurate, experimentally determined values for the solubility in sea water of all but a few compounds and the approximate nature of theoretical calculations which must usually be made using assumed values for the activity coefficients of ions in this complex solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Krauskopf, K. B., Geochim. Cosmochim. Acta, 9, 1 (1956).
Goldberg, E. D., in Chemical Oceanography (edit. by Riley, J. P., and Skirrow, G.), 1, 163 (Academic Press, London, 1965).
Chow, J. T., and Goldberg, E. D., Geochim. Cosmochim. Acta, 20, 192 (1960).
Turekian, K. K., and Johnson, D. G., Geochim. Cosmochim. Acta, 30, 1153 (1966).
Spitsyn, V. I., Torchenkova, E. A., and Glazkova, I. N., Dokl. Akad. Nauk SSSR, 133, 1111 (1960).
Ramette, R. W., and Anderson, O., J. Inorg. Nucl. Chem., 25, 763 (1963).
Bovington, C. H., J. Inorg. Nucl. Chem., 27, 1975 (1965).
Rosseinsky, D. R., Trans. Faraday Soc., 104, 116 (1958).
Bowen, H. J. M., J. Mar. Biol. Assoc. UK, 35, 451 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BURTON, J., MARSHALL, N. & PHILLIPS, A. Solubility of Barium Sulphate in Sea Water. Nature 217, 834–835 (1968). https://doi.org/10.1038/217834a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/217834a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.