Abstract
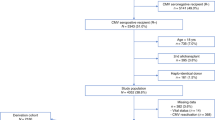
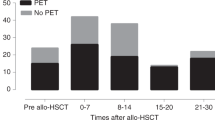
A randomized trial comparing a DNAemia cutoff of 10 000 copies per ml whole blood and first pp65 antigenemia positivity for initiation of preemptive therapy of human cytomegalovirus (HCMV) infection in adult hematopoietic stem cell transplant recipients was completed. DNAemia was chosen for cutoff definition since it is more automatable and standardizable than antigenemia, and more closely reflects the actual viral replication. The primary end point of the study was to compare the number of patients treated in the two arms. A total of 83 patients (42 in the DNAemia, and 41 in the antigenemia arm) were enrolled in the study. The incidence of HCMV infection, as detected by the relevant randomization assay (76% in the DNAemia versus 85% in the antigenemia arm), was comparable in the two arms, whereas the number of patients treated was significantly lower in the DNAemia arm (63 versus 80%, P=0.02). A single patient in the DNAemia arm suffered from biopsy-proven HCMV gastric disease diagnosed in the absence of detectable virus in blood. The incidence of graft-versus-host disease, and transplantation-related mortality did not differ between the two arms. In conclusion, our study shows that the use of a cutoff significantly reduces the number of patients requiring antiviral treatment, thus sparing unnecessary drug administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA . Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 1996; 88: 4063–4071.
Nguyen Q, Champlin R, Giralt S, Rolston K, Raad I, Jacobson K et al. Late cytomegalovirus pneumonia in adult allogeneic blood and marrow transplant recipients. Clin Infect Dis 1999; 28: 618–623.
Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD . Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood 1992; 80: 1358–1364.
Locatelli F, Percivalle E, Comoli P, Maccario R, Zecca M, Giorgiani G et al. Human cytomegalovirus (HCMV) infection in paediatric patients given allogeneic bone marrow transplantation: role of early antiviral treatment for HCMV antigenaemia on patients' outcome. Br J Hematol 1994; 88: 64–71.
Einsele H, Ehninger G, Hebart H, Wittkowski KM, Schuler U, Jahn G et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood 1995; 86: 2815–2820.
Ljungman P, Aschan J, Lewensohn-Fuchs I, Carlens S, Larsson K, Lonnqvist B et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation 1998; 66: 1330–1334.
Enright H, Haake R, Weisdorf D, Ramsay N, McGlave P, Kersey J et al. Cytomegalovirus pneumonia after bone marrow transplantation: risk factors and response to therapy. Transplantation 1993; 55: 1339–1346.
Gerna G, Lilleri D, Baldanti F, Torsellini M, Giorgiani G, Zecca M et al. Human cytomegalovirus immediate-early mRNAemia versus pp65 antigenemia for guiding pre-emptive therapy in children and young adults undergoing hematopoietic stem cell transplantation: a prospective, randomized, open-label trial. Blood 2003; 101: 5053–5060.
Lilleri D, Baldanti F, Gatti M, Rovida F, Dossena L, De Grazia S et al. Clinically-based determination of safe DNAemia cutoff levels for preemptive therapy of human cytomegalovirus infections in solid organ and hematopoietic stem cell transplant recipients. J Med Virol 2004; 73: 412–418.
Gerna G, Lilleri D . Monitoring transplant patients for human cytomegalovirus: diagnostic update. Herpes 2006; 13: 4–11.
Lilleri D, Gerna G, Furione M, Bernardo ME, Giorgiani G, Telli S et al. Use of a DNAemia cut-off for monitoring human cytomegalovirus infection reduces the number of pre-emptively treated children and young adults receiving haematopoietic stem cell transplantation as compared to qualitative pp65-antigenemia. Blood 2007; 10: 2757–2760.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Salvaneschi L, Perotti C, Zecca M, Bernuzzi S, Viarengo G, Giorgiani G et al. Extracorporeal photochemotherapy for treatment of acute and chronic GvHD in childhood. Transfusion 2001; 41: 1299–1305.
Gerna G, Revello MG, Percivalle E, Torsellini M . A 6-h microneutralization assay for human cytomegalovirus antibody by using monoclonal antibodies. Serodiagn Immunother Infect Dis 1990; 4: 243–247.
Gerna G, Vitulo P, Rovida F, Lilleri D, Pellegrini C, Oggionni T et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol 2006; 78: 408–416.
Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-van Dillen PME, van der Nordaa J . Rapid and simple method for purification of nucleic acids. J Clin Microbiol 1990; 28: 495–503.
Gerna G, Revello MG, Percivalle E, Morini F . Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J Clin Microbiol 1992; 30: 1232–1237.
Gerna G, Percivalle E, Torsellini M, Revello MG . Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. J Clin Microbiol 1998; 36: 3585–3589.
Lozza L, Lilleri D, Percivalle E, Fornara C, Comolli G, Revello MG et al. Simultaneous quantification of human cytomegalovirus (HCMV)-specific CD4+ and CD8+ T cells by a novel method using monocyte-derived HCMV-infected immature dendritic cells. Eur J Immunol 2005; 35: 1795–1804.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Klein JP, Rizzo JD, Zhang M-J, Keiding N . Statistical methods for analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant 2001; 28: 909–915.
Gerna G, Lilleri D, Zecca M, Alessandrino EP, Baldanti F, Revello MG et al. Rising antigenemia levels may be misleading in pre-emptive therapy of human cytomegalovirus infection in allogeneic hematopoietic stem cell transplant recipients. Haematologica 2005; 90: 526–533.
Gerna G, Sarasini A, Lilleri D, Percivalle E, Torsellini M, Baldanti F et al. In vitro model for the study of the dissociation of increasing antigenemia and decreasing DNAemia and viremia during treatment of human cytomegalovirus infection with ganciclovir in transplant recipients. J Infect Dis 2003; 188: 1639–1647.
Verkruyse LA, Storch GA, Devine SM, Dipersio JF, Vij R . Once daily ganciclovir as initial pre-emptive therapy delayed until threshold CMV load>or =10 000 copies/ml: a safe and effective strategy for allogeneic stem cell transplant patients. Bone Marrow Transplant 2006; 37: 51–56.
Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F . Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood 2006; 108: 1406–1412.
Gerna G, Zavattoni M, Baldanti F, Sarasini A, Chezzi L, Grossi P et al. Human cytomegalovirus (HCMV) leukoDNAemia correlates more closely with clinical symptoms than antigenemia and viremia in heart and heart-lung transplant recipients with primary HCMV infection. Transplantation 1998; 65: 1378–1385.
Acknowledgements
We thank all the technical staff of the Servizio di Virologia for performing the assays, Daniela Sartori for preparing the paper and Laurene Kelly for revision of English. This work was partially supported by grants from the Ministero della Salute, Fondazione IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo (Ricerca Corrente grant 80541, Ricerca Finalizzata 2003 grant 89269 and Fondazione Cariplo grant 93005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerna, G., Lilleri, D., Caldera, D. et al. Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients. Bone Marrow Transplant 41, 873–879 (2008). https://doi.org/10.1038/sj.bmt.1705986
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705986
Keywords
This article is cited by
-
Scoring system for clinically significant CMV infection in seropositive recipients following allogenic hematopoietic cell transplant: an SFGM-TC study
Bone Marrow Transplantation (2021)
-
A Low Incidence of Cytomegalo Virus Infection Following Allogeneic Hematopoietic Stem Cell Transplantation Despite a High Seroprevalence
Indian Journal of Hematology and Blood Transfusion (2018)
-
The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients
Cellular and Molecular Life Sciences (2015)
-
Pre-emptive antiviral therapy for active CMV infection in adult allo-SCT patients guided by plasma CMV DNAemia quantitation using a real-time PCR assay: clinical experience at a single center
Bone Marrow Transplantation (2013)
-
Differential outcome of neurological HCMV infection in two hematopoietic stem cell transplant recipients
BMC Infectious Diseases (2012)