Abstract
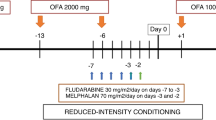
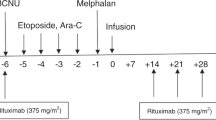
Allogeneic SCT is an effective therapy for lymphoma. Reduced-intensity conditioning (RIC) reduces non-relapse mortality (NRM) associated with myeloablative conditioning but relapse rates are high when performed in active disease. This study was designed to explore the safety and outcome of ibritumomab tiuxetan (Zevalin) combined with RIC in patients with advanced lymphoma. The study included 12 patients, median age 54 years (37–62), with a median of four prior treatments (2–6) and active disease documented on PET–CT. Zevalin 0.4 mCi/kg was given on day −14 and fludarabine combined with BU (n=6) or melphalan (n=6) was started on day −6. GVHD prevention was tapered 3 months after SCT to augment the graft-versus-lymphoma effect. All patients engrafted, a median of 14 days after SCT. Eighty-three percent achieved CR/PR. With a median follow-up of 21 months (12–37), 2-year PFS is 33%. Only three patients relapsed; cumulative incidence 25%. NRM was 42%, predominantly due to acute GVHD. Zevalin–RIC is feasible with consistent engraftment, acceptable organ toxicity, but high rates of acute GVHD. The low incidence of relapse suggests augmented anti-lymphoma effect. Zevalin–RIC merits further study. Better results may be achieved in patients earlier in disease course and with longer duration of immune-suppression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med 1987; 316: 1493–1498.
Shipp MA, Abeloff MD, Antman KH, Carroll G, Hagenbeek A, Loeffler M et al. International consensus conference on high-dose therapy with hematopoietic stem cell transplantation in aggressive non-Hodgkin's lymphomas: report of the jury. J Clin Oncol 1999; 17: 423–429.
Butcher BW, Collins Jr RH . The graft-versus-lymphoma effect: clinical review and future opportunities. Bone Marrow Transplant 2005; 36: 1–17.
Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood 2002; 100: 4310–4316.
van Besien K, Sobocinski KA, Rowlings PA, Murphy SC, Armitage JO, Bishop MR et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 1998; 91: 1178–1184.
Jones RJ, Ambinder RF, Piantadosi S, Santos GW . Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood 1991; 77: 649–653.
Champlin R, Khouri I, Shimoni A, Gajewski J, Kornblau S, Molldrem J et al. Harnessing graft-versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol 2000; 111: 18–29.
Nagler A, Slavin S, Varadi G, Naparstek E, Samuel S, Or R . Allogeneic peripheral blood stem cell transplantation using a fludarabine-based low intensity conditioning regimen for malignant lymphoma. Bone Marrow Transplant 2000; 25: 1021–1028.
Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O'Brien S et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998; 16: 2817–2824.
Escalon MP, Champlin RE, Saliba RM, Acholonu SA, Hosing C, Fayad L et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin's lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol 2004; 22: 2419–2423.
Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood 2004; 104: 3535–3542.
Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood 2004; 104: 3865–3871.
Dean RM, Fowler DH, Wilson WH, Odom J, Steinberg SM, Chow C et al. Efficacy of reduced-intensity allogeneic stem cell transplantation in chemotherapy-refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2005; 11: 593–599.
Gopal AK, Pagel JM, Rajendran JG, Maloney DG, Appelbaum FR, Sorror ML et al. Improving the efficacy of reduced intensity allogeneic transplantation for lymphoma using radioimmunotherapy. Biol Blood Marrow Transplant 2006; 12: 697–702.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005; 23: 1993–2003.
Cheson BD . Radioimmunotherapy of non-Hodgkin lymphomas. Blood 2003; 101: 391–398.
Press OW, Eary JF, Appelbaum FR, Martin PJ, Badger CC, Nelp WB et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med 1993; 329: 1219–1224.
Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin's lymphoma. J Clin Oncol 2005; 23: 461–467.
Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 2005; 106: 2896–2902.
Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin's lymphoma. Exp Hematol 2007; 35: 534–540.
Gopal AK, Rajendran JG, Gooley TA, Pagel JM, Fisher DR, Petersdorf SH et al. High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or=60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol 2007; 25: 1396–1402.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assos 1958; 53: 457–481.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Bar-Natan M, Tsolakis F et al. Comparison between two reduced-intensity conditioning regimens prior to allogeneic stem cell transplantation: Fludarabine and melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine and busulfan (abstract). Blood 2006; 108 (Suppl 1): 835a (abstract 2947).
Knox SJ, Levy R, Miller RA, Uhland W, Schiele J, Ruehl W et al. Determinants of the antitumor effect of radiolabeled monoclonal antibodies. Cancer Res 1990; 50: 4935–4940.
Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood 2006; 107: 2184–2191.
Burke JM, Caron PC, Papadopoulos EB, Divgi CR, Sgouros G, Panageas KS et al. Cytoreduction with iodine-131-anti-CD33 antibodies before bone marrow transplantation for advanced myeloid leukemias. Bone Marrow Transplant 2003; 32: 549–556.
Bunjes D, Buchmann I, Duncker C, Seitz U, Kotzerke J, Wiesneth M et al. Rhenium 188-labeled anti-CD66 (a, b, c, e) monoclonal antibody to intensify the conditioning regimen prior to stem cell transplantation for patients with high-risk acute myeloid leukemia or myelodysplastic syndrome: results of a phase I–II study. Blood 2001; 98: 565–572.
Bethge WA, Wilbur DS, Sandmaier BM . Radioimmunotherapy as non-myeloablative conditioning for allogeneic marrow transplantation. Leuk Lymphoma 2006; 47: 1205–1214.
Gopal AK, Rajendran JG, Pagel JM, Guthrie KA, Maloney DG, Appelbaum FR et al. A phase II trial of 90Y-Ibritumomab Tiuxetan-Based Reduced Intensity Allogeneic Peripheral Blood Stem Cell (PBSC) Transplantation for Relapsed CD20+ B-Cell Non-Hodgkins Lymphoma (NHL). Blood 2006; 108 (Suppl 1): 98a (abstract 316).
Khouri IF, Saliba RM, Hosing C, Valverde R, Erwin WD, Fayad L et al. Efficacy and Safety of Yttrium 90 (90Y) ibritumomab tiuxetan in autologous and nonmyeloablative stem cell transplantation (NST) for relapsed non-Hodgkin's lymphoma (NHL). Blood 2006; 108 (Suppl 1): 98a (abstract 315).
Fietz T, Uharek L, Gentilini C, Muessig A, Rieger K, Marinets O et al. Allogeneic hematopoietic cell transplantation following conditioning with 90Y-ibritumomab-tiuxetan. Leuk Lymphoma 2006; 47: 59–63.
Witzig TE, Molina A, Gordon LI, Emmanouilides C, Schilder RJ, Flinn IW et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer 2007; 109: 1804–1810.
Shimoni A, Hardan I, Avigdor A, Yeshurum M, Raananai P, Ben-Bassat I et al. Rituximab reduces relapse risk after allogeneic and autologous stem cell transplantation in patients with high-risk aggressive non-Hodgkin's lymphoma. Br J Haematol 2003; 122: 457–464.
Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006; 108: 756–762.
Antin JH, Ferrara JL . Cytokine dysregulation and acute graft-versus-host disease. Blood 1992; 80: 2964–2968.
Johansson JE, Brune M, Ekman T . The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell transplantation with reduced intensity conditioning. Bone Marrow Transplant 2001; 28: 737–742.
Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 2003; 102: 756–762.
Khouri IF, Przepiorka D, van Besien K, O'Brien S, Palmer JL, Lerner S et al. Allogeneic blood or marrow transplantation for chronic lymphocytic leukaemia: timing of transplantation and potential effect of fludarabine on acute graft-versus-host disease. Br J Haematol 1997; 97: 466–473.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimoni, A., Zwas, S., Oksman, Y. et al. Ibritumomab tiuxetan (Zevalin) combined with reduced-intensity conditioning and allogeneic stem-cell transplantation (SCT) in patients with chemorefractory non-Hodgkin's lymphoma. Bone Marrow Transplant 41, 355–361 (2008). https://doi.org/10.1038/sj.bmt.1705919
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705919
Keywords
This article is cited by
-
Yttrium-90 ibritumomab tiuxetan plus ATG/TLI for allogeneic hematopoietic cell transplantation in non-Hodgkin lymphoma
Bone Marrow Transplantation (2023)
-
Allogeneic Stem Cell Transplantation for Non-Hodgkin Lymphoma
Current Hematologic Malignancy Reports (2016)
-
Dose-escalated radioimmunotherapy as part of reduced intensity conditioning for allogeneic transplantation in patients with advanced high-grade non-Hodgkin lymphoma
Bone Marrow Transplantation (2012)
-
90Y-ibritumomab tiuxetan followed by reduced-intensity conditioning and allo-SCT in patients with advanced follicular lymphoma
Bone Marrow Transplantation (2011)
-
Current status and future perspectives for yttrium-90 (90Y)-ibritumomab tiuxetan in stem cell transplantation for non-Hodgkin's lymphoma
Bone Marrow Transplantation (2007)