Abstract
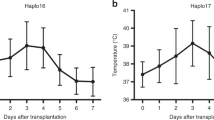
Peg-filgrastim is a form of G-CSF with a sustained duration of action due to self-limited clearance. We administered 6 mg peg-filgrastim to 18 autograft recipients on day +1 after transplantation for hematologic malignancies. Plasma samples were collected at baseline and during transplantation. Hematopoietic recovery and clinical outcomes were compared to the historical data of 54 patients not receiving G-CSF. Patients receiving peg-filgrastim achieved a serum level of 115 000 pg/ml on day +2, 24 h after drug administration. Drug level maintained a plateau until day +8 and, after day +10, declined concomitantly with myeloid recovery. Patients experienced prompt neutrophil recovery: days +9 and +10 to 500 and 1000 neutrophils per microliter, and 4 days with an absolute neutrophil count <100 cells per microliter. Duration of antibiotic therapy was significantly shortened, but we did not observe significant differences in other end points. In conclusion, peg-filgrastim was well tolerated and efficacious, and hastened myeloid recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tarella C, Castellino C, Locatelli F, Caracciolo D, Corradini P, Falda M et al. G-CSF administration following peripheral blood progenitor cell (PBPC) autograft in lymphoid malignancies: evidence for clinical benefits and reduction of treatment costs. Bone Marrow Transplant 1998; 21: 401–407.
Kodo H, Tajika K, Takahashi S, Ozawa K, Asano S, Takaku F . Acceleration of neutrophilic granulocyte recovery after bone marrow transplantation by administration of recombinant human granulocyte colony stimulating factor. Lancet 1988; 2: 38–39.
Sheridan WP, Morstyn G, Wolf M, Dodds A, Lusk J, Maher D et al. Granulocyte-colony stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet 1989; 2: 891–895.
Stahel RA, Jost LM, Cerny T, Pichert G, Honegger H, Tobler A et al. Randomized study of recombinant human granulocyte colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation for high-risk lymphoid malignancies. J Clin Oncol 1994; 12: 1931–1938.
Linch DC, Milligan DW, Winfield DA, Kelsey SM, Johnson SA, Littlewood TJ et al. G-CSF after peripheral blood stem cell transplantation in lymphoma patients significantly accelerated neutrophil recovery and shortened time in hospital: results of a randomized BNLI trial. Br J Haematol 1997; 99: 933–938.
Spitzer G, Adkins DR, Spencer V, Dunphy FR, Petruska PJ, Velasquez WS et al. Randomized study of growth factors post-peripheral-blood stem-cell transplant: neutrophil recovery is improved with modest clinical benefit. J Clin Oncol 1994; 12: 661–670.
Shimazaki C, Oku N, Uchiyama H, Yamagata N, Tatsumi T, Hirata T et al. Effect of granulocyte-colony stimulating factor on hematopoietic recovery after peripheral blood progenitor cell transplantation. Bone Marrow Transplant 1994; 13: 271–275.
Cortelazzo S, Viero P, Bellavita P, Rossi A, Buelli M, Borleri GM et al. Granulocyte-colony stimulating factor following peripheral-blood progenitor transplant in non-Hodgkin's lymphoma. J Clin Oncol 1995; 13: 935–941.
Klumpp TR, Mangan KF, Goldberg SL, Pearlman ES, Macdonald JS . Granulocyte-colony stimulating factors accelerates neutrophil engraftment following peripheral- blood stem cell transplantation:a prospective randomized trial. J Clin Oncol 1995; 13: 1323–1327.
Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA et al. American Society of Clinical Oncology: 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol 2000; 18: 3558–3585.
Colby C, McAfee SL, Finkelstein DM, Spitzer TR . Early vs delayed administration of G-CSF following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 1998; 21: 1005–1010.
Taylor KM, Jagannath S, Spitzer G, Spinolo JA, Tucker SL, Fogel B et al. Recombinant human granulocyte colony-stimulating factor hastens granulocyte recovery after high-dose chemotherapy and autologous bone marrow transplantation in Hodgkin's disease. J Clin Oncol 1989; 7: 1791–1799. Erratum in: J Clin Oncol 1990; 8: 567.
Madero L, Muonz A, Diaz de Heredia A, Martinez A, Badell I, Esquembre C et al. G-CSF after autologous bone marrow transplantation for malignant diseases in children. Spanish Working Party for Bone Marrow Transplantation in Children. Bone Marrow Transplant 1995; 15: 349–351.
Souetre E, Qing W, Penelaud PF . Economic analysis of the use of recombinant human granulocyte colony stimulating factor in autologous bone marrow transplantation. Eur J Cancer 1996; 32A: 1162–1165.
Schmitz N, Ljungman P, Cordonnier C, Kempf C, Linkesch W, Alegre A et al. Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transplant 2004; 34: 955–962.
Piccirillo N, Sica S, Laurenti L, Chiusolo P, La Barbera EO, Sorà F et al. Optimal timing of G-CSF administration after CD34+ immunoselected peripheral blood progenitor cell transplantation. Bone Marrow Transplant 1999; 23: 1245–1250.
Molineux G, Kinstler O, Briddell B, Hartley C, McElroy P, Kerzic P et al. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol 1999; 27: 1724–1734.
Layton JE, Hockman H, Sheridan WP, Morstyn G . Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood 1989; 74: 1303–1307.
Van Der Auwera P, Platzer E, Xu ZX, Schulz R, Feugeas O, Capdeville R et al. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25–8315) in healthy volunteers: comparison with single and multiple daily doses of filgrastim. Am J Hematol 2001; 66: 245–251.
Centers for Disease Control and Prevention (CDC). Centers for Disease Control and Prevention guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mortal Wkly Rep 2002; 51: 27–28.
McQuaker IG, Haynes AP, Anderson S, Stainer C, Owen RG, Morgan GJ et al. Engraftment and molecular monitoring of CD34+ peripheral-blood stem-cell transplants for follicular lymphoma: a pilot study. J Clin Oncol 1997; 15: 2288–2295.
Valteau-Couanet D, Faucher C, Auperin A, Michon J, Milpied N, Boiron JM et al. Cost effectiveness of day 5 G-CSF (Lenograstim) administration after PBSC transplantation: results of a SFGM-TC randomised trial. Bone Marrow Transplant 2005; 36: 547–552.
George S, Yunus F, Case D, Yang BB, Hackett J, Shogan JE et al. Fixed-dose pegfilgrastim is safe and allows neutrophil recovery in patients with non-Hodgkin's lymphoma. Leuk Lymphoma 2003; 44: 1691–1696.
Holmes FA, Jones SE, O’Shaughnessy J, Vukelja S, George T, Savin M et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 2002; 13: 903–909.
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 2002; 20: 727–731.
Johnston E, Crawford J, Blackwell S, Bjurstrom T, Lockbaum P, Roskos L et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol 2000; 18: 2522–2528.
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P et al. International Pegfilgrastim 749 Study Group: a randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003; 14: 29–35.
Vose JM, Crump M, Lazarus H, Emmanouilides C, Schenkein D, Moore J et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol 2003; 21: 514–519.
Jagasia MH, Greer JP, Morgan DS, Mineishi S, Kassim AA, Ruffner KL et al. Pegfilgrastim after high-dose chemotherapy and autologous peripheral blood stem cell transplant: phase II study. Bone Marrow Transplant 2005; 35: 1165–1169.
Laurenti L, Piccioni P, Piccirillo N, Sora F, Chiusolo P, Garzia M et al. Immune recovery of lymphocyte subsets 6 years after autologous peripheral blood stem cell transplantation (PBSCT) for lymphoproliferative diseases. A comparison between NHL, HD and MM in group of 149 patients. Leuk Lymphoma 2004; 45: 2063–2070.
Albo C, de la Fuente J, Ares C, Alonso C, Feteira E . Kinetics and immunophenotypic characterization of circulating hematopoietic progenitor cells after peripheral blood stem cell transplantation. Haematologica 2004; 89: 845–851.
Acknowledgements
This work was supported in part by Associazione Italiana per la Ricerca contro il Cancro (AIRC) Milan, Italy. We are grateful to the nursing staff of the Divisione Ematologia, Policlinico A Gemelli.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piccirillo, N., De Matteis, S., De Vita, S. et al. Kinetics of peg-filgrastim after high-dose chemotherapy and autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 40, 579–583 (2007). https://doi.org/10.1038/sj.bmt.1705772
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705772
Keywords
This article is cited by
-
Pegfilgrastim for PBSC mobilization and autologous haematopoietic SCT
Bone Marrow Transplantation (2009)