Abstract
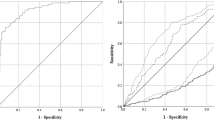
Quantitative cytomegalovirus (CMV) monitoring is still far from being standardized between transplant centers. In the present study, we compared assays for quantitative CMV monitoring using blood cells and plasma. Four hundred and thirty-five consecutive samples from 29 patients with active CMV infection after allogeneic T-cell-depleted hemopoietic stem cell transplantation were tested in parallel using pp65 antigenemia and quantitative CMV polymerase chain reaction (PCR) in blood cells and plasma (COBAS AMPLICOR CMV MONITOR). Although only 142 (53.1%) of 253 positive samples were concordantly identified by all three assays, the number of positive samples detected by each assay was not different and the quantitative values were correlated, provided that nucleic acid (NA) in plasma was isolated by COBAS AmpliPrep and not by the manual protocol. Six (18%) of 34 episodes with active CMV infection were not detected using CMV PCR in plasma; whereas in times of white blood cell aplasia or blast crisis of leukemia, samples with active CMV infection in plasma could not be detected using blood cells. We conclude that CMV monitoring in whole blood could be favorable compared with assays using plasma or blood cells alone. Automated NA isolation could become an attractive tool for a more sensitive and better standardized molecular diagnostics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pass RF . Cytomegalovirus. In: Knipe DM, Howley PM (eds). Fields Virology, 4th edn. Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001, pp 2675–2705.
Ljungman P, Reusser P, de la CR, Einsele H, Engelhard D, Ribaud P et al. Management of CMV infections: recommendations from the infectious diseases working party of the EBMT. Bone Marrow Transplant 2004; 33: 1075–1081.
Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD . Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355: 2032–2036.
Verkruyse LA, Storch GA, Devine SM, DiPersio JF, Vij R . Once daily ganciclovir as initial pre-emptive therapy delayed until threshold CMV load >/=10000 copies/ml: a safe and effective strategy for allogeneic stem cell transplant patients. Bone Marrow Transplant 2006; 37: 51–56.
Boeckh M, Boivin G . Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev 1998; 11: 533–554.
Limaye AP, Huang ML, Leisenring W, Stensland L, Corey L, Boeckh M . Cytomegalovirus (CMV) DNA load in plasma for the diagnosis of CMV disease before engraftment in hematopoietic stem-cell transplant recipients. J Infect Dis 2001; 183: 377–382.
Caliendo AM, Schuurman R, Yen-Lieberman B, Spector SA, Andersen J, Manjiry R et al. Comparison of quantitative and qualitative PCR assays for cytomegalovirus DNA in plasma. J Clin Microbiol 2001; 39: 1334–1338.
Herrmann B, Larsson VC, Rubin CJ, Sund F, Eriksson BM, Arvidson J et al. Comparison of a duplex quantitative real-time PCR assay and the COBAS Amplicor CMV Monitor test for detection of cytomegalovirus. J Clin Microbiol 2004; 42: 1909–1914.
Kalpoe JS, Kroes AC, de J, Schinkel J, de Brouwer CS, Beersma MF et al. Validation of clinical application of cytomegalovirus plasma DNA load measurement and definition of treatment criteria by analysis of correlation to antigen detection. J Clin Microbiol 2004; 42: 1498–1504.
von Müller L, Hampl W, Hinz J, Meisel H, Reip A, Engelmann E et al. High variability between results of different in-house tests for cytomegalovirus (CMV) monitoring and a standardized quantitative plasma CMV PCR assay. J Clin Microbiol 2002; 40: 2285–2287.
Gartner BC, Fischinger JM, Litwicki A, Roemer K, Mueller-Lantzsch N . Evaluation of a new automated, standardized generic nucleic acid extraction method (total nucleic acid isolation kit) used in combination with cytomegalovirus DNA quantification by COBAS AMPLICOR CMV MONITOR. J Clin Microbiol 2004; 42: 3881–3882.
Einsele H, Steidle M, Vallbracht A, Saal JG, Ehninger G, Müller CA . Early occurrence of human cytomegalovirus infection after bone marrow transplantation as demonstrated by the polymerase chain reaction technique. Blood 1991; 77: 1104–1110.
Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol 2004; 42: 1142–1148.
Boivin G, Bélanger R, Delage R, Béliveau C, Demers C, Goyette N et al. Quantitative analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay and the Cobas Amplicor CMV Monitor PCR test after blood and marrow allogeneic transplantation. J Clin Microbiol 2000; 38: 4356–4360.
Boeckh M, Gallez-Hawkins G, Myerson D, Zaia JA, Bowden RA . Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation. Transplantation 1997; 64: 108–113.
Acknowledgements
This work was supported by Roche Diagnostics. The funding source was not involved in the study design, collection, analysis, interpretation of data, the writing of the report and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Müller, L., Hinz, J., Bommer, M. et al. CMV monitoring using blood cells and plasma: a comparison of apples with oranges?. Bone Marrow Transplant 39, 353–357 (2007). https://doi.org/10.1038/sj.bmt.1705593
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705593
Keywords
This article is cited by
-
Diagnostic value of HCMV pp65 antigen detection by FCA for symptomatic and asymptomatic infection: compared to quantification of HCMV DNA and detection of IgM antibody in infants
Medical Microbiology and Immunology (2009)
-
Human cytomegalovirus infection and antiviral immunity in septic patients without canonical immunosuppression
Medical Microbiology and Immunology (2008)
-
Relationship between pp65 antigenemia levels and real-time quantitative DNA PCR for Human Cytomegalovirus (HCMV) management in immunocompromised patients
BMC Infectious Diseases (2007)