Abstract
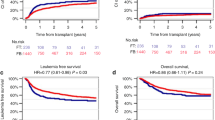
Fludarabine was utilized in the conditioning regimen of 30 adult patients undergoing an allogeneic hematopoietic stem cell transplant. In 18 patients it was combined with full-dose busulfan (FluBu) as a myeloablative regimen and in 12 cases with melphalan (FluMel) as a reduced intensity conditioning (RIC) regimen. Patients in the FluBu group were younger than in the FluMel group (P=0.03). Of 30 patients, 24 received peripheral blood stem cells (PBSC) whereas six patients in the FluBu group received bone marrow cells. The hematological toxicity of each regimen was evaluated by analyzing the kinetics of the neutropenia induced by preparative regimens and the time to recovery of the absolute neutrophils count (ANC) and platelets post transplantation. In PBSC transplants, the median day of severe neutropenia (<500 ANC/μl) occurred on day +6 after the FluBu regimen and on day +3 after FluMel (P=ns), whereas both groups had a duration of severe neutropenia of 9 days and a comparable time for ANC and platelet engraftment. Extra-hematological toxicities were also comparable in the two groups. These findings suggest that the hematological and extra-hematological toxicities induced by fludarabine/full-dose i.v. busulfan are similar to those induced by a standard RIC regimen such as fludarabine/melphalan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zinzani PL, Lauria F, Rondelli D, Benfenati D, Raspadori D, Bocchia M et al. Fludarabine: an active agent in the treatment of previously-treated and untreated low-grade non-Hodgkin's lymphoma. Ann Oncol 1993; 4: 575–578.
Lenz G, Hiddemann W, Dreyling M . The role of fludarabine in the treatment of follicular and mantle cell lymphoma. Cancer 2004; 101: 883–893.
Czuczman MS, Koryzna A, Mohr A, Stewart C, Donohue K, Blumenson L et al. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol 2005; 23: 694–704.
Hallek M, Schmitt B, Wilhelm M, Busch R, Krober A, Fostitsch HP et al. Fludarabine plus cyclophosphamide is an efficient treatment for advanced chronic lymphocytic leukaemia (CLL): results of a phase II study of the German CLL Study Group. Br J Haematol 2001; 114: 342–348.
Terenzi A, Aristei C, Aversa F, Perruccio K, Chionne F, Raymondi C et al. Efficacy of fludarabine as an immunosuppressor for bone marrow transplantation conditioning: preliminary results. Transplant Proc 1996; 28: 3101.
Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP et al. Allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in intermediate or high risk patients with myelofibrosis with myeloid metaplasia. Blood 2005; 105: 4115–4119.
Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol 2004; 22: 2172–2176.
Alessandrino EP, Bernasconi P, Colombo AA, Caldera D, Bonfichi M, Pagnucco G et al. Thiotepa and fludarabine (TT-FLUDA) as conditioning regimen in poor candidates for conventional allogeneic hemopoietic stem cell transplant. Ann Hematol 2001; 80: 521–524.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Kroger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br J Haematol 2005; 128: 690–697.
Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood 2004; 104: 3535–3542.
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 1998; 339: 1186–1193.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857–864.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8: 468–476.
Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 2003; 102: 820–826.
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, Naumann R et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol 2001; 114: 944–950.
Devine SM, Hoffman R, Verma A, Shah R, Bradlow BA, Stock W et al. Allogeneic blood cell transplantation following reduced-intensity conditioning is effective therapy for olde patients with myelofibrosis with myeloid metaplasia. Blood 2002; 99: 2255–2258.
Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant 1999; 23: 1055–1060.
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Shimoni A, Bielorai B, Toren A, Hardan I, Avigdor A, Yeshurun M et al. Intravenous busulfan-based conditioning prior to allogeneic hematopoietic stem cell transplantation: myeloablation with reduced toxicity. Exp Hematol 2003; 31: 428–434.
Williams CB, Day SD, Reed MD, Copelan EA, Bechtel T, Leather HL et al. Dose modification protocol using intravenous busulfan (Busulfex) and cyclophosphamide followed by autologous or allogeneic peripheral blood stem cell transplantation in patients with hematologic malignancies. Biol Blood Marrow Transplant 2004; 10: 614–623.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chunduri, S., Dobogai, L., Peace, D. et al. Comparable kinetics of myeloablation between fludarabine/full-dose busulfan and fludarabine/melphalan conditioning regimens in allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 38, 477–482 (2006). https://doi.org/10.1038/sj.bmt.1705480
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705480
Keywords
This article is cited by
-
Renal dysfunction within 90 days of FluBu4 predicts early and late mortality
Bone Marrow Transplantation (2019)
-
The impact of CD34+ cell dose on engraftment after SCTs: personalized estimates based on mathematical modeling
Bone Marrow Transplantation (2014)
-
Promising Role of Reduced-Toxicity Hematopoietic Stem Cell Transplantation (PART-I)
Stem Cell Reviews and Reports (2012)
-
Comorbidity index does not predict outcome in allogeneic myeloablative transplants conditioned with fludarabine/i.v. busulfan (FluBu4)
Bone Marrow Transplantation (2011)
-
Reliability of a pretransplant i.v. BU test dose performed 2 weeks before myeloablative FluBu conditioning regimen
Bone Marrow Transplantation (2010)