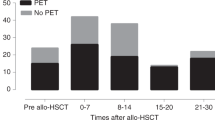
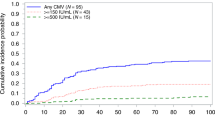
Abstract
Quantitative polymerase chain reaction (QPCR) for cytomegalovirus (CMV) is emerging as the preferred screening method for detection of CMV viremia in patients following allogeneic bone marrow and peripheral blood stem cell transplant. However, there are currently no universally accepted QPCR treatment thresholds at which to start pre-emptive therapy. We report here results of a pre-emptive therapy strategy using ganciclovir (GCV) 5 mg/kg initiated once daily (ODG) delayed till a threshold CMV load of ⩾10 000 copies/ml whole blood in clinically stable patients. Sixty-nine at risk patients underwent allogeneic stem cell transplant. 48/69 (70%) patients had an initial episode of CMV viremia. 5/48 (10%) cleared viremia without requiring treatment. 28/43 (65%) patients requiring treatment initiated treatment with ODG. 17/28 (61%) patients successfully cleared CMV viremia on ODG, 10/28 (36%) patients required dose escalation to twice daily GCV for increasing viral loads. There were two cases of CMV disease (colitis) and no deaths due to CMV disease in patients initiating treatment with ODG. We conclude delaying pre-emptive therapy with ODG until whole blood QPCR⩾10 000 copies/ml is a safe and effective strategy for CMV viremia after allogeneic stem cell transplant in clinically stable patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Winston DJ, Ho WG, Lin CH, Bartoni K, Budlinger MD, Gale RP et al. Intraveneous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann Intern Med 1987; 106: 12–18.
Meyers JD, Flournoy N, Thomas ED . Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis 1986; 153: 478–488.
Verdonck LF, Dekker AW, Rozenberg-Arska M, van den Hoek MR . A risk-adapted approach with a short course of ganciclovir to prevent cytomegalovirus (CMV) pneumonia in CMV-seropositive recipients of allogeneic bone marrow transplants. Clin Infect Dis 1997; 24: 901–907.
Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA . Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 1996; 88: 4063–4071.
Goodrich JM, Mori M, Gleaves CA, DuMond C, Cays M, Ebling DF et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med 1991; 325: 1601–1607.
Kanda Y, Mineishi S, Saito T, Seo S, Saito A, Ohnishi M et al. Pre-emptive therapy against cytomegalovirus (CMV) disease guided by CMV antigenemia assay after allogeneic hematopoietic stem cell transplantation: a single-center experience in Japan. Bone Marrow Transplant 2001; 27: 437–444.
Gleaves CA, Smith TF, Shuster EA, Pearson GR . Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol 1984; 19: 917–919.
Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD . Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood 1992; 80: 1358–1364.
Qamruddin AO, Oppenheim BA, Guiver M, Mutton KJ, Chopra R . Screening for cytomegalovirus (CMV) infection in allogeneic bone marrow transplantation using a quantitative whole blood polymerase chain reaction (PCR) method: analysis of potential risk factors for CMV infection. Bone Marrow Transplant 2001; 27: 301–306.
Mori T, Okamoto S, Watanabe R, Yajima T, Iwao Y, Yamazaki R et al. Dose-adjusted preemptive therapy for cytomegalovirus disease based on real-time polymerase chain reaction after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 29: 777–782.
Schulenburg A, Watkins-Riedel T, Greinix HT, Rabitsch W, Loidolt H, Keil F et al. CMV monitoring after peripheral blood stem cell and bone marrow transplantation by pp65 antigen and quantitative PCR. Bone Marrow Transplant 2001; 28: 765–768.
Einsele H, Ehninger G, Hebart H, Wittowski KM, Schuler U, Jahn G et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood 1995; 86: 2815–2820.
Griscelli F, Barrois M, Chauvin S, Lastere S, Bellet D, Bourhis JH . Quantification of human cytomegalovirus DNA in bone marrow transplant recipients by real-time PCR. J Clin Microbiol 2001; 39: 4362–4369.
Hassan-Walker AF, Kidd IM, Sabin C, Sweny P, Griffiths PD, Emery VC . Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG). J Med Virol 1999; 58: 182–187.
Vij R, Khoury H, Brown R, Goodnough LT, Devine SM, Blum W et al. Low-dose short-course intravenous ganciclovir as pre-emptive therapy for CMV viremia post allo-PBSC transplantation. Bone Marrow Transplant 2003; 32: 703–707.
Sanchez JE, Storch GA . Multiplex, quantitative, real-time PCR assay for cytomegalovirus and human DNA. J Clin Microbiol 2002; 40: 2381–2386.
Yakushiji K, Gondo H, Kamezaki K, Shigematsu K, Hayashi S, Kuroiwa M et al. Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transplant 2002; 29: 599–606.
Haddad N, Galbraith J, Vij R, DiPersio JF, Shannon J, Duncan W et al. PCR versus culture for diagnosis and management of CMV in allogeneic stem cell transplant (SCT) recipients. Abstracts of the 40th Annual Meeting of the Infectious Diseases Society of America 2002, p 142 (Abstract 304).
Gor D, Sabin C, Prentice HG, Vyas N, Man S, Griffiths PD et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant 1998; 21: 597–605.
Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD et al. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355: 2032–2036.
Peggs KS, Preiser W, Kottaridis PD, McKeag N, Brink NS, Tedder RS et al. Extended routine polymerase chain reaction surveillance and pre-emptive antiviral therapy for cytomegalovirus after allogeneic transplantation. Br J Haematol 2000; 111: 782–790.
Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002; 99: 1159–1164.
Trenschel R, Ross S, Husing J, Ottinger H, Elmaagacli A, Roggendorf M et al. Reduced risk of persisting cytomegalovirus pp65 antigenemia and cytomegalovirus interstitial pneumonia following allogeneic PBSCT. Bone Marrow Transplant 2000; 25: 665–672.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verkruyse, L., Storch, G., Devine, S. et al. Once daily ganciclovir as initial pre-emptive therapy delayed until threshold CMV load ⩾10000 copies/ml: a safe and effective strategy for allogeneic stem cell transplant patients. Bone Marrow Transplant 37, 51–56 (2006). https://doi.org/10.1038/sj.bmt.1705213
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705213
Keywords
This article is cited by
-
Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients
Bone Marrow Transplantation (2013)
-
A randomized controlled trial of plasma real-time PCR and antigenemia assay for monitoring CMV infection after unrelated BMT
Bone Marrow Transplantation (2010)
-
Preemptive therapy with ganciclovir 5 mg/kg once daily for cytomegalovirus infection after unrelated cord blood transplantation
Bone Marrow Transplantation (2008)
-
Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients
Bone Marrow Transplantation (2008)
-
The effect of quantification standards used in real-time CMV PCR assays on guidelines for initiation of therapy in allogeneic stem cell transplant patients
Bone Marrow Transplantation (2007)