Summary:
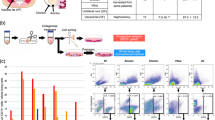
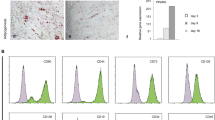
Human mesenchymal stem cells (hMSCs) are excellent candidates for ex vivo gene transfer and cell therapy in various systems. However, hMSCs are mortal somatic cells, and thus invariably enter an irreversible growth arrest after a finite number of cell divisions in culture. It has been proposed that this is due to telomere shortening. In this study, pGRN145 plasmid containing human telomerase reverse transcriptase (hTRT) was introduced into fetal dermis-derived hMSCs. Single-cell clones positive for telomerase activity and hTRT mRNA were selected and expanded. Single-cell-derived hTRT+ cells could be expanded rapidly in vitro and passaged up to 70 doublings without showing senescence. FACScan flow cytometer showed that hTRT+ cells were positive for CD29, CD44, CD105, and CD166, while CD31, CD45, CD34, vWF, and HLA-DR were negative. Under suitable conditions, hTRT+ cells have the ability of multiple lineage differentiation, including bone, fat, and nerve. Furthermore, transplantation of hTRT+ cells could protect NOD/SCID mice from lethal irradiation. Thus, these cells may be an ideal cell source for promoting hematopoietic recovery after radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Friedenstein AJ, Deriglazova UF, Kulagina NN et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974; 2: 83–92.
Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147.
Filvaroff EH, Derynck R . Induction of myogenesis in mesenchymal cells by myoD depends on their degree of differentiation. Dev Biol 1996; 178: 459–471.
Makino S, Fukuda K, Miyoshi S et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999; 103: 697–705.
Zhao LR, Duan WM, Reyes M et al. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 2002; 174: 11–20.
Young RG, Butler DL, Weber W et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 1998l; 16: 406–413.
Majumdar MK, Thiede MA, Haynesworth SE et al. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000; 9: 841–848.
Horwitz EM, Prockop DJ, Fitzpatrick LA et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313.
Maitra B, Szekely E, Gjini K et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant 2004; 33: 597–604.
Minguell JJ, Conget P, Erices A . Biology and clinical utilization of mesenchymal progenitor cells. Braz J Med Biol Res 2000; 33: 881–887.
Fang B, Liao L, Zhao RC et al. Multipotency of Flk1CD34 progenitors derived from human fetal bone marrow. J Lab Clin Med 2004; 143: 230–240.
Fang B, Shi M, Zhao RC et al. Multiorgan engraftment and multilineage differentiation by human fetal bone marrow Flk1+/CD31−/CD34− Progenitors. J Hematother Stem Cell Res 2003; 12: 603–613.
Guo H, Fang BJ, Zhao RC et al. Hemangioblastic characteristics of fetal bone marrow–derived Flk1+CD31CD34 cells. Exp Hematol 2003; 31: 650–658.
Li M, Lianming L, Zhao RC et al. Murine dermal mesenchymal stem cells enhance recovery of hematopoiesis after sublethal irradiation. Stem Cell Cell Ther 2003; 1: 44–48.
Serrano M, Blasco MA . Putting the stress on senescence. Curr Opin Cell Biol 2001; 13: 748–753.
Sherr CJ, DePinho RA . Cellular senescence: mitotic clock or culture shock? Cell 2000; 102: 407–410.
Zimmermann S, Voss M, Kaiser S et al. Lack of telomerase activity in human mesenchymal stem cells. Leukemia 2003; 17: 1146–1149.
Wright WE, Shay JW, Piatyszek MA . Modifications of a telomeric repeat amplification protocol TRAP; result in increased reliability, linearity and sensitivity. Nucleic Acids Res 1995; 23: 3794–3795.
Kim NW, Piatyszek MA, Prowse KR et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–2015.
Reyes M, Lund T, Lenvik T et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001; 98: 2615–2625.
Allers C, Sierralta WD, Neubauer S et al. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation 2004; 78: 503–508.
Bodnar AG, Ouellette M, Frolkis M et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279: 349–532.
Deans RJ, Moseley AB . Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000; 28: 875–884.
Whetton AD, Graham GJ . Homing and mobilization in the stem cell niche. Trends Cell Biol 1999; 9: 233–238.
Verfaillie CM . Adhesion receptors as regulators of the hematopoietic process. Blood 1998; 92: 2609–2612.
O’Flaherty E, Sparrow R, Szer J . Bone marrow stromal function from patients after bone marrow transplantation. Bone Marrow Transplant 1995; 15: 207–212.
Cilloni D, Carlo-Stella C, Falzetti C et al. Limited engraftment capacity of bone marrow-derived mesenchymal cells following T-cell-depleted hematopoietic stem cell transplantation. Blood 2000; 96: 3637–3643.
Koc O, Lazarus H . Mesenchymal stem cells heading to the clinic. Bone Marrow Transplant 2001; 27: 235–239.
Toksoz D, Zsebo K, Smith K et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane bound and secreted forms of the human homologue of the steel gene product stem cell factor. Proc Natl Acad Sci USA 1992; 89: 7350–7354.
Majumdar MK, Thiede MA, Mosca JD et al. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 1998; 176: 186–192.
Anklesaria P, Kase K, Glowacki J et al. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci USA 1987; 1987: 7681–7685.
Nolta J, Hanley M, Kohn D . Sustainedhuman hematopoiesis in immunode cient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood 1994; 83: 3041–3051.
Noort WA, Kruisselbrink AB, Anker PS et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 (+) cells in NOD/SCID mice. Exp Hematol 2002; 30: 870–878.
Almeida-Porada G, Porada C, Tran N, Zanjani E . Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood 2000; 95: 3620–3627.
Angelopoulou M, Novelli E, Grove J et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis andmegakaryocytopoiesis in NOD/SCID mice. Exp Hematol 2003; 31: 413–420.
Liu L, DiGirolamo CM, Navarro PA et al. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Experimental Cell Research 2004; 294: 1–8.
Burns JS, Abdallah BM, Guldberg P et al. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res 2005; 65: 3126–3135.
Coffin J, Hughes S, Varmus H . Retroviruses. Cold Spring Harbor Laboratory Press: New York, 1997, p 843.
Sandmaier B, Strob R, Kniley J et al. Evidence of allogeneic stromal engraftment in bone marrow using canine mesenchymal stem cells. Blood 1998; 92: 116a.
Devine S, Bartholomew A, Mahmud N et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 2001; 29: 244–255.
Wong GH, Goeddel DV . Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science 1988; 242: 941–944.
Neta R, Sztein MB, Oppenheim JJ et al. The in vivo effects of interleukin 1. I. Bone marrow cells are induced to cycle after administration of interleukin 1. J Immunol 1987; 139: 1861–1866.
Acknowledgements
We thank Li Ma, Shaoguang Yang, and Mingxia Shi for technical help. This study was supported by grants from the ‘863 Projects’ of Ministry of Science and Technology of PR China (No. 2002AA205061); from China Medical Board of New York Inc.: Stem Cell Biology, Engineering (Grant #01-748); from National Natural Science Foundation of China (No. 30070284); from National Key Project for Basic Research of China (No. 001CB5099); from Peking Ministry of Science and Technology (No. 2002-489). RCH Zhao is a Cheung Kong Scholar in PR China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Z., Liao, L., Cao, Y. et al. Establishment and properties of fetal dermis-derived mesenchymal stem cell lines: plasticity in vitro and hematopoietic protection in vivo. Bone Marrow Transplant 36, 355–365 (2005). https://doi.org/10.1038/sj.bmt.1705062
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705062
Keywords
This article is cited by
-
Eminent Sources of Adult Mesenchymal Stem Cells and Their Therapeutic Imminence
Stem Cell Reviews and Reports (2017)
-
Human Fibroblasts Share Immunosuppressive Properties with Bone Marrow Mesenchymal Stem Cells
Journal of Clinical Immunology (2010)
-
Mesenchymale Stammzellen der Haut
Der Hautarzt (2010)
-
Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model
Cell Research (2008)