Summary:
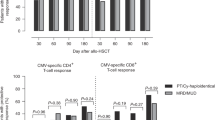
Peripheral blood stem cell transplantation after reduced-intensity conditioning (RIC-PBSCT) regimen is an alternative to conventional regimens with less immediate toxicity. Since immune recovery is of crucial importance for the control of infections, we retrospectively studied the recovery of T-, B- and NK cell subsets in 20 consecutive patients undergoing RIC-PBSCT. We also studied the thymic output using T-cell receptor excision circle assay. Engraftment was rapid and few infectious complications were seen: three early (before 2.5 months) cases of asymptomatic cytomegalovirus reactivation, two late Gram-negative bacterial infections and no fungal infection. While CD4+ T-cell reconstitution was slow, CD8+ T-cell counts were close to normal values at 4 months. Median CD19+ B-cell counts reached normal values at 11 months. Rapid CD56+ NK cell reconstitution was noticed as early as 1.5 months. Low T-cell receptor excision circle numbers and preponderance of memory-type subsets among T cells further suggested that CD8+ T-cell reconstitution resulted predominantly from peripheral expansion and that thymic-dependent reconstitution was severely impaired. In conclusion, large peripheral T-cell expansion may compensate for late thymic-dependent lymphopoiesis, and may, with other factors such as NK and B-cell reconstitution and careful antiinfectious prophylaxis, help limit the incidence of severe infections after RIC-PBSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Storb RF, Champlin R, Riddell SR et al. Non-myeloablative transplants for malignant disease. Hematology 2001; 91: 375–391.
Feinstein L, Storb R . Nonmyeloablative hematopoietic cell transplantation. Curr Opin Oncol 2001; 13: 95–100.
Slavin S, Nagler A, Naparstek E et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Badros A, Barlogie B, Morris C et al. High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood 2001; 97: 2574–2579.
Childs R, Clave E, Contentin N et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood 1999; 94: 3234–3241.
Junghanss C, Marr KA . Infectious risks and outcomes after stem cell transplantation: are nonmyeloablative transplants changing the picture? Curr Opin Infect Dis 2002; 15: 347–353.
Junghanss C, Marr KA, Carter RA et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant 2002; 8: 512–520.
Hagen EA, Stern H, Porter D et al. High rate of invasive fungal infections following nonmyeloablative allogeneic transplantation. Clin Infect Dis 2003; 36: 9–15.
Ljungman P . Immune reconstitution and viral infections after stem cell transplantation. Bone Marrow Transplant 1998; 21 (Suppl. 2): S72–S74.
Boeckh M, Nichols WG . The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004; 103: 2003–2008.
Junghanss C, Boeckh M, Carter RA et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood 2002; 99: 1978–1985.
Storek J, Gooley T, Witherspoon RP et al. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4T cell counts. Am J Hematol 1997; 54: 131–138.
Mackall CL, Granger L, Sheard MA et al. T-cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood 1993; 82: 2585–2594.
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant 1995; 16: 413–425.
Dumont-Girard F, Roux E, van Lier RA et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood 1998; 92: 4464–4471.
Weinberg K, Blazar BR, Wagner JE et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood 2001; 97: 1458–1466.
Storek J, Dawson MA, Storer B et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001; 97: 3380–3389.
Champlin RE, Schmitz N, Horowitz MM et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood 2000; 95: 3702–3709.
Ottinger HD, Beelen DW, Scheulen B et al. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood 1996; 88: 2775–2779.
Petersen SL, Ryder LP, Bjork P et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant 2003; 32: 65–72.
Boeckh M, Gooley TA, Myerson D et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 1996; 88: 4063–4071.
Ascioglu S, Rex JH, de Pauw B et al. Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
Douek DC, Vescio RA, Betts MR et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000; 355: 1875–1881.
Thiede C, Florek M, Bornhauser M et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant 1999; 23: 1055–1060.
Hamann D, Baars PA, Rep MHG et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997; 186: 1407–1418.
Storek J, Joseph A, Dawson MA et al. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation 2002; 73: 1154–1158.
Fallen PR, McGreavey L, Madrigal JA et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 2003; 32: 1001–1014.
Kong FK, Chen CL, Six A et al. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci USA 1999; 96: 1536–1540.
Heitger A, Neu N, Kern H et al. Essential role of the thymus to reconstitute naïve (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood 1997; 90: 850–857.
Johannisson A, Festin R . Phenotype transition of CD4+ T cells from CD45RA to CD45RO is accompanied by cell activation and proliferation. Cytometry 1995; 19: 343–352.
Hazenberg MD, Borghans JA, de Boer RJ et al. Thymic output: a bad TREC record. Nat Immunol 2003; 4: 97–99.
Bahceci E, Epperson D, Douek D et al. Early reconstitution of the T-cell repertoire after non-myeloablative peripheral blood stem cell transplantation is from post-thymic T-cell expansion and is unaffected by graft-versus-host disease of mixed chimerism. Br J Haematol 2003; 122: 934–943.
Mohty M, Gaugler B, Faucher C et al. Recovery of lymphocyte and dendritic cell subsets following reduced intensity allogeneic bone marrow transplantation. Hematology 2002; 7: 157–164.
Hamann D, Kostense S, Wolthers KC et al. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int Immunol 1999; 11: 1027–1033.
Rowbottom AW, Garland RJ, Lepper MW et al. Functional analysis of the CD8+CD57+ cell population in normal healthy individuals and matched unrelated T-cell-depleted bone marrow transplant recipients. Br J Haematol 2000; 110: 315–321.
Wang EC, Borysiewicz LK . The role of CD8+, CD57+ cells in human cytomegalovirus and other viral infections. Scand J Infect Dis Suppl 1995; 99: 69–77.
Maris M, Boeckh M, Storer B et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol 2003; 31: 941–952.
Busca A, Lovisone E, Aliberti S et al. Immune reconstitution and early infectious complications following nonmyeloablative hematopoietic stem cell transplantation. Hematology 2003; 8: 303–311.
Mohty M, Jacot W, Faucher C et al. Infectious complications following allogeneic HLA-identical sibling transplantation with antithymocyte globulin-based reduced intensity preparative regimen. Leukemia 2003; 17: 2168–2177.
Mossad SB, Avery RK, Longworth DL et al. Infectious complications within the first year after nonmyeloablative allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2001; 28: 491–495.
Mohty M, Faucher C, Vey N et al. High rate of secondary viral and bacterial infections in patients undergoing allogeneic bone marrow mini-transplantation. Bone Marrow Transplant 2000; 26: 251–255.
Mohty M, Mohty AM, Blaise D et al. Cytomegalovirus-specific immune recovery following allogeneic HLA-identical sibling transplantation with reduced-intensity preparative regimen. Bone Marrow Transplant 2004; 33: 839–846.
Acknowledgements
We are indebted to all the clinical staff of the Bone Marrow Transplantation Unit of the Department of Hematology and the technical staff of the Immunology and Molecular Biology laboratories of the EFS Bourgogne/Franche-Comté for their help and their contribution to this work. This study was supported by grants from the associations ‘Cent pour Sang la Vie’ and ‘Moelle Partage et Vie’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larosa, F., Marmier, C., Robinet, E. et al. Peripheral T-cell expansion and low infection rate after reduced-intensity conditioning and allogeneic blood stem cell transplantation. Bone Marrow Transplant 35, 859–868 (2005). https://doi.org/10.1038/sj.bmt.1704889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704889
Keywords
This article is cited by
-
Dynamics of recent thymic emigrants in pediatric recipients of allogeneic hematopoetic stem cell transplantation
Bone Marrow Transplantation (2022)
-
Autoimmune cytopenias (AIC) following allogeneic haematopoietic stem cell transplant for acquired aplastic anaemia: a joint study of the Autoimmune Diseases and Severe Aplastic Anaemia Working Parties (ADWP/SAAWP) of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2020)
-
Initial fluconazole prophylaxis may not be required in adults with acute leukemia or myelodysplastic/myeloproliferative disorders after reduced intensity conditioning peripheral blood stem cell allogeneic transplantation
Annals of Hematology (2015)
-
Targeting natural killer cells and natural killer T cells in cancer
Nature Reviews Immunology (2012)
-
Rapid helper T-cell recovery above 200 × 106/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation
Bone Marrow Transplantation (2006)