Summary:
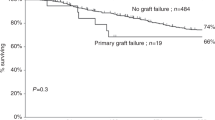
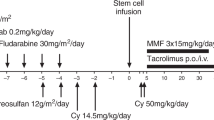
Haemopoietic stem cell transplants (HSCT) cure increasing numbers of primary immunodeficiencies (PID): residual recipient T-cell function increases risk of incomplete or decreasing immune reconstitution, which may resolve following a second, unconditioned, infusion from the same donor (boost infusion). We assessed the outcome of 20 boost infusions in 19/139 patients transplanted for PID patients at our centre since 1987. Boost infusion was given 64–1226 days after the original HSCT. Follow-up was 4–124 months. In all, 12 of 19 patients cleared viral infection (6), or showed sustained increase in donor chimerism, T- and B-cell numbers and function, or other markers (6). In 7/12 patients, immunoglobulin replacement has been discontinued. Four were partially successful with stable low-level chimerism (two patients) or improved T-cell function, but not B cell function (two patients). Four failed with no change in donor chimerism or cell number. No significant association with donor source, T-cell depletion, conditioning regimen, boost infusion stem cell dose or time from original HSCT to boost was found. One patient developed grade III acute graft-versus-host disease despite cyclosporine, and one developed severe pneumonitis; both have recovered. Boost infusion was successful or partially successful in 84% of patients. The risk of adverse effects is low.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Antoine C, Muller S, Cant A et al, for the European Group for Blood and Marrow Transplantation; European Society for Immunodeficiency Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet 2003; 361: 553–560.
Filipovich AH, Stone JV, Tomany SC et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott–Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood 2001; 97: 1598–1603.
Gennery AR, Khawaja K, Veys P et al. Treatment of CD40 ligand deficiency by hematopoietic stem cell transplantation: a survey of the European experience, 1993–2002. Blood 2004; 103: 1152–1157.
Gross TG, Filipovich AH, Conley ME et al. Cure of X-linked lymphoproliferative disease (XLP) with allogeneic hematopoietic stem cell transplantation (HSCT): report from the XLP registry. Bone Marrow Transplant 1996; 17: 741–744.
Seger RA, Gungor T, Belohradsky BH et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood 2002; 100: 4344–4350.
Baud O, Goulet O, Canioni D et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med 2001; 344: 1758–1762.
Buckley RH, Schiff SE, Schiff RI et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med 1999; 340: 508–516.
Kline RM, Stiehm ER, Cowan MJ . Bone marrow ‘boosts’ following T cell-depleted haploidentical bone marrow transplantation. Bone Marrow Transplant 1996; 17: 543–548.
Amrolia P, Gaspar HB, Hassan A et al. Nonmyeloablative stem cell transplantation for congenital immunodeficiencies. Blood 2000; 96: 1239–1246.
Zaucha JM, Gooley T, Bensinger WI et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood 2001; 98: 3221–3227.
Quinones RR . Hematopoietic engraftment and graft failure after bone marrow transplantation. Am J Pediatr Hematol Oncol 1993; 15: 3–17.
Rosenfeld CS, Rybka WB, Weinbaum D et al. Late graft failure due to dual bone marrow infection with variants A and B of human herpesvirus-6. Exp Hematol 1995; 23: 626–629.
Steffens HP, Podlech J, Kurz S et al. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hemopoietin gene expression in recipient stroma. J Virol 1998; 72: 5006–5015.
Johnston RE, Geretti AM, Prentice HG et al. HHV-6-related secondary graft failure following allogeneic bone marrow transplantation. Br J Haematol 1999; 105: 1041–1043.
Green A, Clarke E, Hunt L et al. Children with acute lymphoblastic leukemia who receive T-cell-depleted HLA mismatched marrow allografts from unrelated donors have an increased incidence of primary graft failure but a similar overall transplant outcome. Blood 1999; 94: 2236–2246.
Remberger M, Ringden O, Ljungman P et al. Booster marrow or blood cells for graft failure after allogeneic bone marrow transplantation. Bone Marrow Transplant 1998; 22: 73–78.
Rao K, Amrolia PJ, Jones A et al. Improved survival after unrelated donor bone marrow transplant in children with primary immunodeficiency using a reduced intensity conditioning regime. Blood 2004; 105: 879–885.
Min CK, Kim DW, Lee JW et al. Additional stem cell therapy for graft failure after allogeneic bone marrow transplantation. Acta Haematol 2000; 104: 185–192.
Wolff SN . Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant 2002; 29: 545–552.
Antin JH, Childs R, Filipovich AH et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2001; 7: 473–485.
Yamaguchi K, Ariga T, Yamada M et al. Mixed chimera status of 12 patients with Wiskott–Aldrich syndrome (WAS) after hematopoietic stem cell transplantation: evaluation by flow cytometric analysis of intracellular WAS protein expression. Blood 2002; 100: 1208–1214.
Ozsahin H, Le Deist F, Benkerrou M et al. Bone marrow transplantation in 26 patients with Wiskott–Aldrich syndrome from a single center. J Pediatr 1996; 129: 238–244.
Lang P, Klingebiel T, Schumm M et al. Correction of persistent thrombocytopenia by a boost of CD133+ selected stem cells in a patient transplanted for Wiskott–Aldrich syndrome 10 years ago. Bone Marrow Transplant 2004; 33: 879–880.
Acknowledgements
We thank Dawn Barge at the Regional Immunology Laboratory, Newcastle upon Tyne NHS Trust for the lymphocyte phenotyping and Tony Jackson at the Northern Regional Genetics service for chimerism analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slatter, M., Bhattacharya, A., Abinun, M. et al. Outcome of boost haemopoietic stem cell transplant for decreased donor chimerism or graft dysfunction in primary immunodeficiency. Bone Marrow Transplant 35, 683–689 (2005). https://doi.org/10.1038/sj.bmt.1704872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704872
Keywords
This article is cited by
-
CD34 Stem Cell Boost in Pediatric Allogeneic Stem Cell Transplant Recipients: A Case Series and Review of Literature
Clinical Hematology International (2023)
-
Poor graft function can be durably and safely improved by CD34+-selected stem cell boosts after allogeneic unrelated matched or mismatched hematopoietic cell transplantation
Journal of Cancer Research and Clinical Oncology (2015)
-
Long-term outcome of non-ablative booster BMT in patients with SCID
Bone Marrow Transplantation (2013)
-
Stable low-level donor-cell engraftment in a patient with X-linked lymphoproliferative disease following matched unrelated allo-SCT
Bone Marrow Transplantation (2011)
-
Partially corrected X-linked severe combined immunodeficiency: long-term problems and treatment options
Immunologic Research (2009)