Summary:
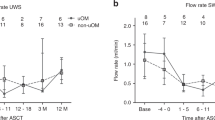
Pilocarpine hydrochloride has been reported to increase salivation and decrease oral mucositis in patients receiving head and neck radiotherapy, but there is only one report of its use in a cancer chemotherapy patient population. This prospective, double-blinded, randomized, placebo-controlled trial was undertaken to determine the efficacy of pilocarpine for the moderation of oral mucositis during autologous blood stem cell transplantation. Subjects were randomized to receive a 5 mg tablet of pilocarpine, or a placebo, during and following chemotherapy. Subjects were seen every other day and evaluated for gingival, oral, and oropharyngeal mucositis; nutrition; oral hygiene; eating; speaking; sleeping; pain at rest and/or with swallowing; and mouth dryness. We recorded the mean and highest scores and duration of problems, along with white blood cell counts and differentials, and the use of systemic narcotics for oral mucosal pain. We enrolled and randomized 36 subjects, and there were no statistically or clinically significant differences for the primary outcome of severity of mucositis and no clinically significant differences in any of the other outcome measures. Pilocarpine has no benefit for the moderation of the incidence, severity, or duration of mucositis in patients receiving autologous blood stem cell transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Juttner CA, To LB . Autologous peripheral blood stem cell transplantation: potential advantages, practical considerations and initial clinical results. In: Atkinson K (ed.). Clinical Bone Marrow Transplantation. Cambridge University Press: London, 1994, pp 142–152.
Heimdahl A, Mattsson T, Dahllöf G et al. The oral cavity as a port of entry for early infections in patients treated with bone marrow transplantation. Oral Surg Oral Med Oral Pathol 1989; 68: 711–716.
Connolly SF, Lockhart PB, Sonis ST . Severe oral hemorrhage and sepsis following bone marrow transplant failure. Oral Surg Oral Med Oral Pathol 1983; 56: 483–486.
Elting LS, Bodey GP, Keefe BH . Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis 1992; 14: 1201–1207.
Perry MC . Genitourinary, gastrointestinal, endocrine, nervous system, and coagulation complications. In: Armitage JO, Antman KH (eds). High-Dose Cancer Therapy. Pharmacology, Hematopoietins, Stem Cells. Williams and Wilkins: Baltimore, 1995, pp 609–618.
Schubert MM, Sullivan KM, Izutsu KT et al. Oral complications of bone-marrow transplantation. In: Peterson DE, Sonis ST (eds). Oral Complications of Cancer Chemotherapy. Martinus Nijhoff Publishing: Boston, 1983, pp 93–112.
Woo S, Sonis ST, Monopoli MM et al. A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 1993; 72: 1612–1617.
Chapko MK, Syrjala KL, Schilter L et al. Chemoradiotherapy toxicity during bone marrow transplantation: time course and variation in pain and nausea. Bone Marrow Transplant 1989; 4: 181–186.
Schubert MM, Peterson DE, Flournoy N et al. Oral and pharyngeal herpes simplex virus infection after allogeneic bone marrow transplantation: analysis of factors associated with infection. Oral Surg Oral Med Oral Pathol 1990; 7: 286–293.
Sonis ST, Oster G, Fuchs H et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 2001; 19: 2201–2205.
Bearman SI, Appelbaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 1988; 6: 1562–1568.
Elad S, Garfunkel AA, Or R et al. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer 2003; 11: 674–677.
Guggenheimer J, Verbin RS, Appel BN . Clinicopathologic effects of cancer chemotherapeutic agents on human buccal mucosa. Oral Surg Oral Med Oral Pathol 1977; 44: 58–63.
Lockhart PB, Sonis ST . Alterations in the oral mucosa caused by chemotherapeutic agents: a histologic study. J Dermatol Surg Oncol 1981; 7: 1019–1025.
Sonis ST . Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 1998; 34: 39–43.
Sonis ST, Van Vugt AG, Brien JPO et al. Transforming growth factor-â3 mediated modulation of cell cycling and attenuation of 5-fluorouracil induced oral mucositis. Oral Oncol 1997; 33: 47–54.
Sonis ST . The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral Biol Med 2002; 13: 380–389.
Tabak LA . In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 1995; 57: 547–564.
Almstahl A, Wikstrom M . Oral microflora in subjects with reduced salivary secretion. J Dent Res 1999; 78: 1410–1416.
Bardow A, Moe D, Nyvad B et al. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch Oral Biol 2000; 45: 1–12.
Epstein JB, Tsang AH, Warkentin D et al. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94: 39–44.
Jensen SB, Pedersen AM, Reibel J et al. Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer 2003; 11: 207–225.
Pedersen AM, Bardow A, Jensen SB et al. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis 2002; 8: 117–129.
Köstler WJ, Hejna M, Wenzel C et al. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin 2001; 51: 290–315.
Gordon B, Spadinger A, Hodges E et al. Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis after hematopoietic stem-cell transplantation. J Clin Oncol 1994; 12: 1917–1922.
Epstein JB, Schubert MM . Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis, and management. Oncology 2003; 17: 1767–1792.
Clarkson JE, Worthington HV, Eden OB . Interventions for preventing oral mucositis or oral candidiasis for patients with cancer receiving chemotherapy (excluding head and neck cancer). The Cochrane Database of Systematic Reviews 2003; 1.
Rocke LK, Loprinzi CL, Lee JK et al. A randomized clinical trial of two different durations of oral cryotherapy for prevention of 5-fluorouracil-related stomatitis. Cancer 1993; 72: 2234–2238.
Cascinu S, Fedeli A, Fedeli SL et al. Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur J Cancer B Oral Oncol 1994; 30B: 234–236.
Fox PC, Atkinson JC, Macynski AA et al. Pilocarpine treatment of salivary gland hypofunction and dry mouth (xerostomia). Arch Intern Med 1991; 151: 1149–1152.
Wiseman LR, Faulds D . Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia. Drugs 1995; 49: 143–156.
Vivino FB, Al-Hashimi I, Khan Z et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: a randomized placebo-controlled fixed-dose multicenter trial. Arch Intern Med 1999; 159: 174–181.
Greenspan D, Daniels TE . Effectiveness of pilocarpine in postradiation xerostomia. Cancer 1987; 59: 1123–1125.
Johnson JT, Ferretti GA, Nethery WJ et al. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med 1993; 329: 390–395.
LeVeque FG, Montgomery M, Potter D et al. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol 1993; 11: 1124–1131.
Rieke JW, Hafermann MD, Johnson JT et al. Oral pilocarpine for radiation-induced xerostomia: integrated efficacy and safety results from two prospective randomized clinical trials. Int J Radiat Oncol Biol Phys 1995; 31: 661–669.
Horiot JC, Lipinski F, Schraub S et al. Post-radiation severe xerostomia relieved by pilocarpine: a prospective French cooperative study. Radiother Oncol 2000; 55: 233–239.
Zimmerman RP, Mark RJ, Tran LM et al. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. Int J Radiat Oncol Biol Phys 1997; 37: 571–575.
Valdez IH, Wolff A, Atkinson JC et al. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer 1993; 71: 1848–1851.
Haddad P, Karimi M . A randomized, double-blind, placebo-controlled trial of concomitant pilocarpine with head and neck irradiation for prevention of radiation-induced xerostomia. Radiother Oncol 2002; 64: 29–32.
LeVeque F, Fontanesi J, Davi S et al. Salivary gland sheltering using concurrent pilocarpine (PC) in irradiated head and neck cancer patients. Proceedings of the Association of Surgical Clinical Oncologists 15, p 516, 1996 (Abstract).
Warde P, O’Sullivan B, Aslanidis J et al. A phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002; 54: 9–13.
Nagler RM, Nagler A . Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: a sialometrical study. Bone Marrow Transplant 1999; 23: 1007–1011.
LeVeque F, Dansey R, Klein B . Use of concurrent oral pilocarpine (OP) to treat mucositis during bone marrow transplantation (BMT): a pilot study. J Clin Oncol 1997; 16: 108a (proceedings) (Abstract).
Awidi A, Homsi U, Kakail RI et al. Double-blind, placebo-controlled cross-over study of oral pilocarpine for the prevention of chemotherapy-induced oral mucositis in adult patients with cancer. Eur J Cancer 2001; 37: 2010–2014.
Tardieu C, Cowen D, Thirion X et al. Quantitative scale of oral mucositis associated with autologous bone marrow transplantation. Eur J Cancer B Oral Oncol 1996; 32B: 381–387.
Landis JR, Koch GG . The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174.
Miller AB, Hoogstraten B, Staquet M et al. Reporting results of cancer treatment. Cancer 1981; 47: 207–214.
Dens F, Boogaerts M, Boute P et al. Caries-related salivary microorganisms and salivary flow rate in bone marrow recipients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81: 38–43.
Amerongen AV, Veerman EC . Saliva – the defender of the oral cavity. Oral Dis 2002; 8: 12–22.
Kaplan MD, Baum BJ . The functions of saliva. Dysphagia 1993; 8: 225–229.
Mandel ID . The role of saliva in maintaining oral homeostasis. J Am Dent Assoc 1989; 119: 298–304.
Baum BJ, Bodner L, Fox PC et al. Therapy-induced dysfunction of salivary glands: implications for oral health. Spec Care Dentist 1985; 5: 274–277.
DeStefano F, Anda RF, Kahn HS et al. Dental disease and risk of coronary heart disease and mortality. BMJ 1993; 306: 688–691.
Mansson-Rahemtulla B, Techanitiswad T, Rahemtulla F et al. Analysis of salivary components in leukemia patients receiving chemotherapy. Oral Surg Oral Med Oral Pathol 1992; 73: 35–46.
Wolff A, Moreira JE, Marmary Y et al. Lack of acute effects of methotrexate on rat parotid salivary gland function. Arch Oral Biol 1989; 34: 109–115.
McBride RK, Siegel IA . Effect of methotrexate on protein and amylase secretion by rat parotid and submandibular salivary glands. Arch Oral Biol 1988; 33: 245–249.
Dens F, Boogaerts M, Boute P et al. Quantitative determination of immunological components of salivary gland secretion in transplant recipients. Bone Marrow Transplant 1996; 17: 421–423.
Jankovic L, Jelic S, Filipovic-Ljeskovic I et al. Salivary immunoglobulins in cancer patients with chemotherapy-related oral mucosa damage. Eur J Cancer Part B, Oral Oncol 1995; 31B: 160–165.
Mandel ID . The functions of saliva. J Dent Res 1987; 66: 623–627.
McCarthy GM, Awde JD, Ghandi H et al. Risk factors associated with mucositis in cancer patients receiving 5-fluorouracil. Oral Oncol 1998; 34: 484–490.
Harrison T, Bigler L, Tucci M et al. Salivary slgA concentrations and stimulated whole saliva flow rates among women undergoing chemotherapy for breast cancer: an exploratory study. Spec Care Dentist 1998; 18: 109–112.
Patterson AJ, Ritschel WA, Zellner D et al. Methotrexate serum and saliva concentrations in patients. Int J Clin Pharmacol, Therapy, Toxicol 1981; 19: 381–385.
Ishii E, Yamada S, Higuchi S et al. Oral mucositis and salivary methotrexate concentration in intermediate-dose methotrexate therapy for children with acute lymphoblastic leukemia. Med Pediatr Oncol 1989; 17: 429–432.
Bressolle F, Jacquet JM, Galtier M et al. Doxorubicin and doxorubicinol plasma concentrations and excretion in parotid saliva. Cancer Chemother Pharmacol 1992; 30: 215–218.
Milano G, Thyss A, Santini J et al. Salivary passage of 5-fluorouracil during continuous infusion. Cancer Chemother Pharmacol 1989; 24: 197–199.
Oliff A, Bleyer WA, Poplack DG . Methotrexate-induced oral mucositis and salivary methotrexate concentrations. Cancer Chemother Pharmacol 1979; 2: 225–226.
Parulekar W, Mackenzie R, Bjarnason G et al. Scoring oral mucositis. Oral Oncol 1998; 34: 63–71.
Schubert MM, Williams BE, Lloid ME et al. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 1992; 69: 2469–2477.
Sonis ST, Eilers JP, Epstein JB et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer 1999; 85: 2103–2113.
Donnelly JP, Muus P, Schattenberg A et al. A scheme for daily monitoring of oral mucositis in allogeneic BMT recipients. Bone Marrow Transplant 1992; 9: 409–413.
McGuire DB, Peterson DE, Muller S et al. The 20 item oral mucositis index: reliability and validity in bone marrow and stem cell transplant patients. Cancer Invest 2002; 20: 893–903.
Scientific Advisory Committee of the Medical Outcomes Trust. Instrument Review Criteria, Medical Outcomes Trust Bulletin 1995; September: 1–4.
Papas AS, Sherrer YS, Charney M et al. Treatment of dry mouth and dry eye symptoms in Sjögren's syndrome patients with oral pilocarpine – a randomized, placebo-controlled, dose adjustment study. J Clin Rheumatol 2004; 10: 169–177.
Acknowledgements
We acknowledge Dr Dorothy Keefe for her critical reviews of the manuscript, and Dr Howell Sasser for his help with data analysis. This study was funded by the Charlotte-Mecklenburg Health Services Foundation, Carolinas Medical Center, Charlotte, NC 28239, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lockhart, P., Brennan, M., Kent, M. et al. Randomized controlled trial of pilocarpine hydrochloride for the moderation of oral mucositis during autologous blood stem cell transplantation. Bone Marrow Transplant 35, 713–720 (2005). https://doi.org/10.1038/sj.bmt.1704820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704820
Keywords
This article is cited by
-
The effect of hematopoietic stem cell transplantation on patient-reported subjective oral dryness: a systematic review focusing on prevalence, severity and distress
Supportive Care in Cancer (2023)
-
Effect of photobiomodulation on the severity of oral mucositis and molecular changes in head and neck cancer patients undergoing radiotherapy: a study protocol for a cost-effectiveness randomized clinical trial
Trials (2019)
-
Systematic review of miscellaneous agents for the management of oral mucositis in cancer patients
Supportive Care in Cancer (2013)